Sol Gel-Derived SBA-16 Mesoporous Material
Abstract
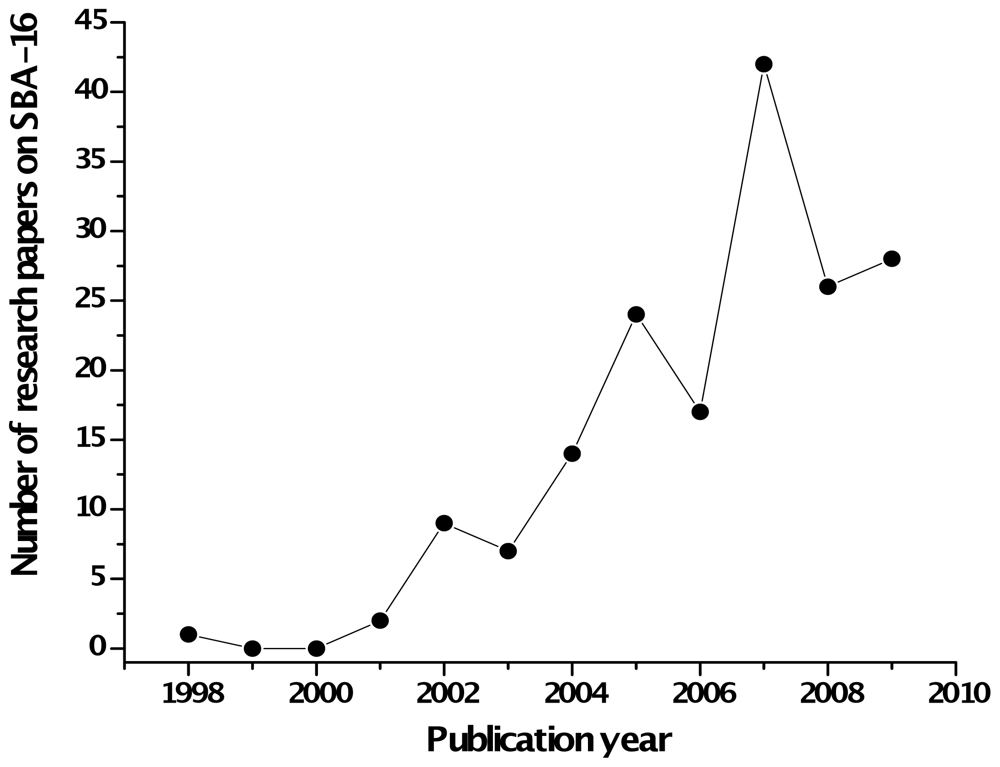
:1. Introduction
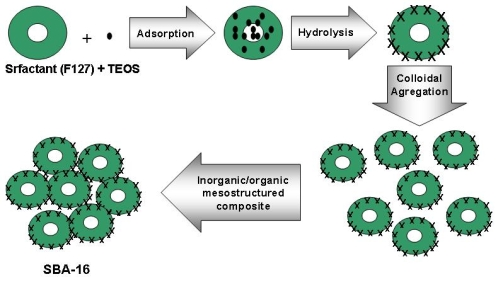
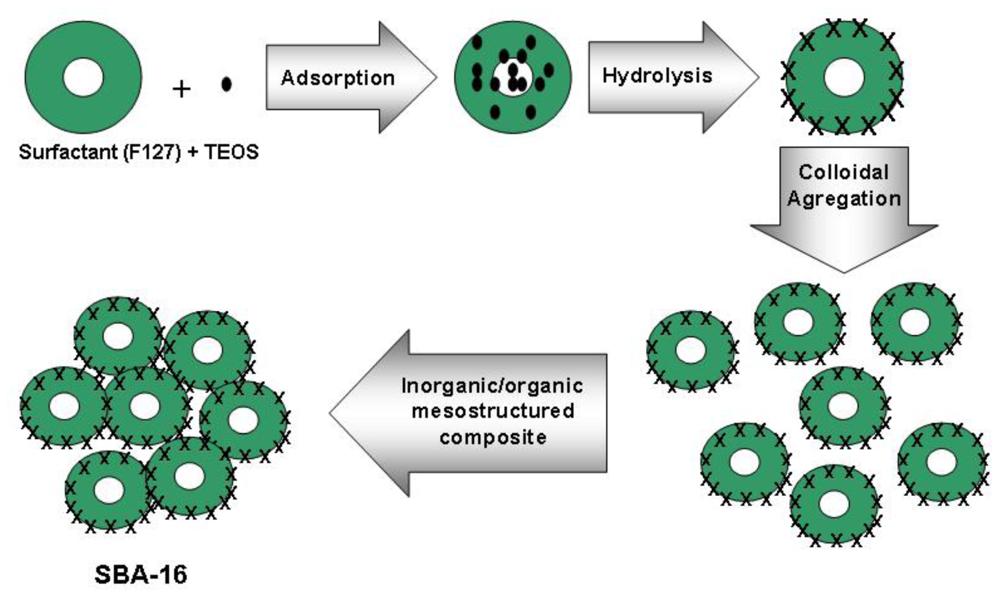
2. Synthesis Mechanism
2.1. The Sol-Gel Process
2.2. The Template Pathway
2.3. Removal of Template
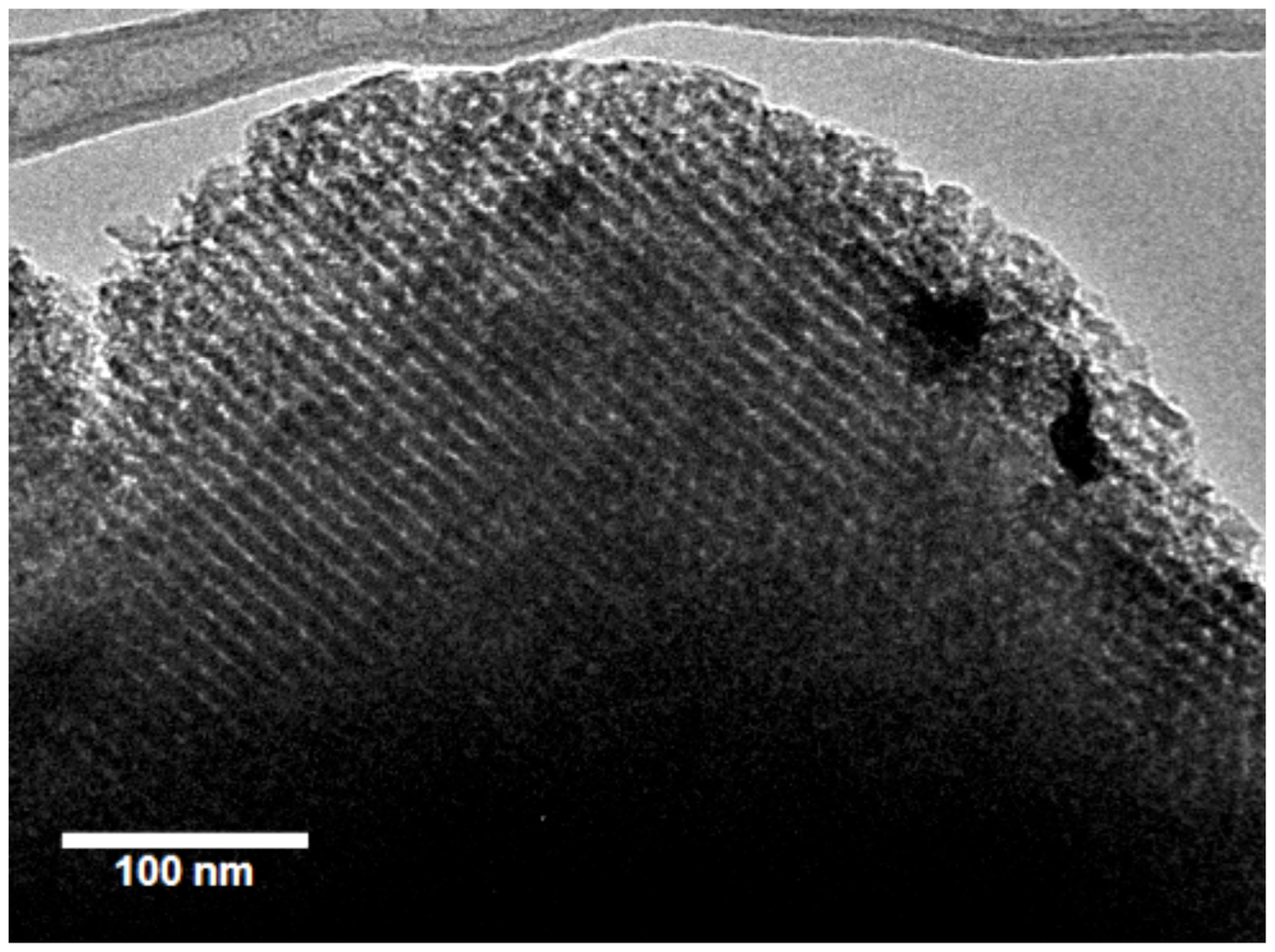
3. Synthesis Methods
4. Applications
4.1. Catalysts
4.2. Functionalization
4.3. Metals Incorporation
4.4. Environmental
4.5. Template
4.6. Electronics
5. Conclusions and Future Prospects
References
- Wang, Y; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev 2007, 107, 2821–2860. [Google Scholar]
- Beck, JS; Vartul, JC; Roth, WJ; Leonowicz, ME; Kresge, CT; Schmitt, KD; Chu, CT-W; Olsen, DH; Sheppard, EW; McCullen, SB; Higgins, JB; Schlenker, JL. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc 1992, 114, 10834–10843. [Google Scholar]
- Kresge, CT; Leonowicz, ME; Roth, WJ; Vartuil, JC; Beck, JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar]
- Zhao, D; Feng, J; Huo, Q; Melosh, N; Fredrickson, GH; Chmelka, BF; Stucky, GD. Triblock copolymer synthesis of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar]
- Zhao, D; Huo, Q; Feng, J; Chmelka, BF; Stucky, GD. Nonionic triblock and star diblock copolymer and oligomeric surfactant synthesis of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc 1998, 120, 6024–6036. [Google Scholar]
- Stevens, WJJ; Lebeau, K; Mertens, M; van Tendeloo, G; Cool P;Vansant, EF. Investigation of the morphology of the mesoporous SBA-16 and SBA-15 materials. J. Phys. Chem. B 2006, 110, 9183–9187. [Google Scholar]
- Sun, H; Tang, Q; Du, Y; Liu, X; Chen, Y; Yang, Y. Mesostructured SBA-16 with excellent hydrothermal, thermal and mechanical stabilities: Modified synthesis and its catalytic application. J. Colloid Interface Sci 2009, 333, 317–323. [Google Scholar]
- Sakamoto, Y; Kaneda, M; Terasaki, O; Zhao, DY; Kim, JM; Stucky, G; Shim, HJ; Ryoo, R. Direct imaging of the pores and cages of three-dimensional mesoporous materials. Nature 2000, 408, 449–453. [Google Scholar]
- Ravikovitch, PI; Neimark, AV. Density functional theory of adsorption in spherical cavities and pores size characterization of templated nanoporous silicas with cubic and three-dimensional hexagonal structures. Langmuir 2002, 18, 1550–1560. [Google Scholar]
- Ravikovitch, PI; Neimark, AV. Experimental confirmation of different mechanisms of evaporation from ink-bottle type pores: Equilibrium, pore blocking, and cavitation. Langmuir 2002, 18, 9830–9837. [Google Scholar]
- Hench, LL; West, KJ. The sol-gel process. Chem. Rev 1990, 90, 33–72. [Google Scholar]
- Gobin, OC. SBA-16 Materials Synthesis, Diffusion and Sorption Properties. Seminar Thesis, Laval University, Quebec, Canada, 2006. [Google Scholar]
- Huo, QS; Margolese, DI; Ciesla, U; Feng, PY; Gier, TE; Sieger, P; Leon, R; Petroff, PM; Schuth, F; Stucky, GD. Generalized synthesis of periodic surfactant/inorganic composite materials. Nature 1994, 368, 317–321. [Google Scholar]
- Bagshaw, SA; Prouzet, E; Pinnavaia, TJ. Templating of mesoporous molecular sieves by nonionic polyethylene oxide surfactans. Science 1995, 269, 1242–1244. [Google Scholar]
- Tanev, PT; Pinnavaia, TJ. A neutral templating route to mesoporous molecular sieves. Science 1995, 267, 865–867. [Google Scholar]
- Kruk, M; Jaroniec, M; Ko, CH; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater 2000, 12, 1961–1968. [Google Scholar]
- Yang, CM; Zibrowius, B; Schmidt, W; Schuth, F. Consecutive generation of mesopores and micropores in SBA-15. Chem. Mater 2003, 15, 3739–3741. [Google Scholar]
- Yang, CM; Schmidt, W; Kleitz, F. Pore topology control of three-dimensional large pore cubic silica mesophases. J. Mater. Chem 2005, 15, 5112–5114. [Google Scholar]
- Grudzien, RM; Grabicka, BE; Jaroniec, M. Efective method to removal of polymeric template from SBA-16 silica combining extraction and temperature-controlled calcinations. J. Mater. Chem 2006, 16, 819–823. [Google Scholar]
- Gallis, KW; Landry, CC. Rapid calcination of nanostructured silicate composites by microwave irradiation. Adv. Mater 2001, 13, 23–26. [Google Scholar]
- Tian, B; Liu, X; Yu, C; Gao, F; Luo, Q; Xie, S; Tu, B; Zhao, D. Microwave assisted template removal of siliceous porous materials. Chem. Commun 2002, 1186–1187. [Google Scholar]
- Hozumi, A; Yokogawa, Y; Kameyama, T; Hiraku, K; Sugimura, H; Takai, O; Okido, M. Photocalcination of mesoporous silica films using vacuum ultraviolet light. Adv. Mater 2000, 12, 985–987. [Google Scholar]
- Huang, L; Poh, C; Ng, SC; Hidajat, K; Kawi, S. Preparation of supported mesoporous thin films concerning template removal by supercritical fluid extraction. Langmuir 2005, 21, 1171–1174. [Google Scholar]
- Voort, PVD; Benjelloun, M; Vansant, EF. Rationalization of the synthesis of SBA-16: Controlling the micro- and mesoporosity. J. Phys. Chem. B 2002, 106, 9027–9032. [Google Scholar]
- Cheng, C-F; Lin, Y-C; Cheng, H-H; Chen, H-C. The effect and model of silica concentrations on physical properties and particle sizes of three dimensional SBA-16 nanoporous material. Chem. Phys. Lett 2003, 382, 496–501. [Google Scholar]
- Wang, L; Fan, J; Tian, B; Yang, H; Yu, C; Tu, B; Zhao, D. Synthesis and characterization of small pore thick-walled SBA-16 templated by oligomeric surfactant with ultra-long hydrophilic chains. Microporous Mesoporous Mater 2004, 67, 135–141. [Google Scholar]
- Cheng, C-F; Lin, Y-C; Cheng, H-H; Liu, S-M; Sheu, H-S. Rapid crystallization of high quality cubic sillica SBA-16 nanoporous material. Chem. Lett 2004, 33, 262–263. [Google Scholar]
- Kim, T-W; Ryoo, R; Kruk, M; Gierszal, KP; Jaroniec, M; Kamiya, S; Teresaki, O. Tailoring the pore structure of SBA-16 silica molecular sieve through the use of copolymer blends and control of synthesis temperature and time. J. Phys. Chem. B 2004, 108, 11480–11489. [Google Scholar]
- Kipkemboi, P; Fogden, A; Alfredsson, V; Flodstrom, K. Triblock copolymers as templates in mesoporous silica formation: Structural dependence on polymer chain length and synthesis temperature. Langmuir 2001, 17, 5398–5402. [Google Scholar]
- Grudzien, RM; Grabicka, BE; Jaroniec, M. Effective method for removal of polymeric template from SBA-16 silica combining extraction and temperature-controlled calcination. J. Mater. Chem 2006, 16, 819–823. [Google Scholar]
- Yamada, T; Zhou, H; Asai, K; Honma, I. Pore size controlled mesoporous silicate powder prepared by triblock copolymer templates. Mater. Lett 2002, 56, 93–96. [Google Scholar]
- Li, D; Guan, X; Song, J; Di, Y; Zhang, D; Ge, X; Zhao, L; Xiao, F-H. Highly efficient synthesis of ordered mesoporous silica materials with controllable microporosity using surfactant mixtures as templates. Colloid Surface A: Physicochem. Eng. Aspect 2006, 272, 194–202. [Google Scholar]
- Mesa, M; Sierra, L; Patarin, J; Guth, JL. Morphology and porosity characteristics control of SBA-16 mesoporous silica. Effect of the triblock surfactant Pluronic F127 degradation during the synthesis. Solid State Sci 2005, 7, 990–997. [Google Scholar]
- Lin, C-L; Pang, Y-S; Chao, M-C; Chen, B-C; Lin, H-P; Tang, C-Y; Lin, C-Y. Synthesis of SBA-16 and SBA-15 mesoporous silica crystal templated with neutral block copolymer surfactants. J. Phys. Chem. Solids 2008, 69, 415–419. [Google Scholar]
- Chen, B-C; Chao, M-C; Lin, H-P; Mou, C-Y. Faceted single crystals of mesoporous SBA-16 from a ternary surfactant system: Surface roughening model. Microporous Mesoporous Mater 2005, 81, 241–249. [Google Scholar]
- Kleitz, F; Kim, TW; Ryoo, R. Phase domain of the cubic Im3m mesoporous silica in the EO106PO70EO106–Butanol-H2O system. Langmuir 2006, 22, 440–445. [Google Scholar]
- Yu, C; Tian, B; Fan, J; Stucky, GD; Zhao, D. Nonionic block copolymer synthesis of large pore-cubic mesoporous single crystals by use of inorganic salts. J. Am. Chem. Soc 2002, 124, 4556–4557. [Google Scholar]
- Yu, C; Fan, J; Tian, B; Zhao, D. Morphology development of mesoporous materials: A colloidal phase separation mechanism. Chem. Mater 2004, 16, 889–898. [Google Scholar]
- Mesa, M; Sierra, L; Guth, J-L. Contribution to the study of the formation mechanism of mesoporous SBA-15 and SBA-16 type silica particles in aqueous acid solutions. Microporous Mesoporous Mater 2008, 112, 338–350. [Google Scholar]
- Murakami, Y; Yamakita, S; Okubo, T; Maruyama, S. Single-walled carbon nanotubes catalytically grown from mesoporous silica thin film. Chem. Phys. Lett 2003, 375, 393–398. [Google Scholar]
- Shi, K-Y; Ji, C-Y; Xin, B-F; Xu, M; Fu, H-G. Carbon nanotubes filled with Fe in SBA-16 thin film of mesoporous silica. Acta Chim. Sinica 2004, 62, 2270–2272. [Google Scholar]
- Shi, K; Chi, Y; Yu, H; Xin, B; Fu, H. Controlled growth of mesostructured crystalline iron oxide nanowires and Fe-filled carbon nanotube arrays templated by mesoporous silica SBA-16 film. J. Phys. Chem. B 2005, 109, 2546–2551. [Google Scholar]
- Shi, K-Y; Li, L; Yang, Y; Jin, X-Q; Shao, S-Z; Wang, H-Y; Fu, H-G. PEI chemical modified multi-walled carbon nanotubes and NO adsorption behavior. J. Harbin Inst. Technol 2006, 38, 2199–2202. [Google Scholar]
- Klimova, T; Lizama, L; Amezcua, JC; Roquero, P; Terrés, E; Navarrete, J; Domínguez, JM. New NiMo catalysts supported on Al-containing SBA-16 for 4,6-DMDBT hydrodesulfurization Effect of the alumination method. Catal. Today 2004, 98, 141–150. [Google Scholar]
- Amezcua, JC; Lizama, L; Salcedo, C; Puente, I; Domínguez, JM; Klimova, T. NiMo catalysts supported on titania-modified SBA-16 for 4,6-dimethyldibenzothiophene hydrodesulfurization. Catal. Today 2005, 107–108, 578–588. [Google Scholar]
- Ma, N; Ji, S-F; Wu, P-Y; Hu, L-H; Nie, P-Y. Preparation, characterization and catalytic activity performance for hydrodesulfurization of WxC/SBA-16 catalysts. Acta Phys.-Chim. Sinica 2007, 23, 1189–1194. [Google Scholar]
- Huirache-Acuña, R; Pawelec, B; Rivera-Muñoz, E; Nava, R; Espino, J; Fierro, JLG. Comparison of the morphology and HDS activity of ternary Co-Mo-W supported on P-modified SBA-15 and SBA-16 substrates. Appl. Catal. B: Environ 2009, 92, 168–184. [Google Scholar]
- Aburto, J; Ayala, M; Bustos-Jaimes, I; Montiel, C; Terrés, E; Domínguez, JM; Torres, E. Stability and catalytic properties of chloroperoxidase immobilized on SBA-16 mesoporous materials. Microporous Mesoporous Mater 2005, 83, 193–200. [Google Scholar]
- Yang, MH; Li, J; Yang, J; Liu, Z; Yang, Q; Li, C. Asymmetric reactions on chiral catalysts entrapped within a mesoporous cage. Chem. Commun 2007, 1086–1088. [Google Scholar]
- Yang, H; Zhang, L; Su, W; Yang, Q; Li, C. Asymmetric ring-opening of epoxides on chiral Co(Salen) catalyst synthesized in SBA-16 through the “ship in a bottle” strategy. J. Catal 2007, 248, 204–212. [Google Scholar]
- Yang, H; Zhang, L; Zhong, L; Yang, Q; Li, C. Enhanced cooperative activation effect in the hydrolytic kinetic resolution of epoxides on [Co(salen)] catalysts confined in nanocages. Angew. Chem. Int. Ed 2007, 46, 6985–6989. [Google Scholar]
- Kim, Y-S; Guo, X-F; Kim, G-J. Highly active new chiral Co(III) salen catalysts immobilized by electrostatic interaction with sulfonic acid linkages on ordered mesoporous SBA-16 silica. Chem. Commun 2009, 28, 4296–4298. [Google Scholar]
- Bordoloi, A; Amrute, AP; Halligudi, SB. [Ru(salen)(NO)] complex encapsulated in mesoporous SBA-16 as catalyst for hydrogenation of ketones. Catal. Commun 2008, 10, 45–48. [Google Scholar]
- Yang, H; Zhang, L; Wang, P; Yang, P; Yang, Q; Li, C. The enantioselective cyanosilylation of aldehydes on a chiral VO(Salen) complex encapsulated in SBA-16. Green Chem 2009, 11, 257–264. [Google Scholar]
- Kataoka, S; Endo, A; Harada, A; Inagi, Y; Ohmori, T. Characterization of mesoporous catalyst supports on microreactor walls. Appl. Catal. A: Gen 2008, 342, 107–112. [Google Scholar]
- Hwang, YK; Kim, D-K; Mamman, AS; Park, S-E; Chang, J-S. Fabrication of nanodispersed Pt-Sn/SBA-16 catalysts and their catalytic applications. Chem. Lett 2007, 36, 186–187. [Google Scholar]
- Hwang, YK; Mamman, AS; Kim, D-K; Park, S-E; Chang, J-S. Enhancement of catalytic activity in dehydrogenation of n-dodecane over nanostructured Pt-Sn/SBA-16 catalysts by microwave drying. Res. Chem. Intermed 2008, 34, 755–760. [Google Scholar]
- Zhang, L; Deng, J; Dai, H; Au, CT. Binary Cr-Mo oxide catalysts supported on MgO-coated polyhedral three-dimensional mesoporous SBA-16 for the oxidative dehydrogenation of iso-butane. Appl. Catal. A: Gen 2009, 354, 72–81. [Google Scholar]
- Yang, H; Han, X; Li, G; Wang, Y. N-Heterocyclic carbine palladium complex supported on ionic liquid-modified SBA-16: An efficient and highly recyclable catalyst for the Suzuki and Heck reactions. Green Chem 2009, 11, 1184–1193. [Google Scholar]
- Nava, R; Pawelec, B; Castaño, P; Álvarez-Galván Loricera, CV; Fierro, JLG. Upgrading of bio-liquids on different mesoporous silica-supported CoMo catalysts. Appl. Catal. B: Environ 2009, 92, 154–167. [Google Scholar]
- Dong, Y; Yuan, F; Zhu, Y; Zhao, L; Cai, Z. Characterization and catalytic properties of mesoporous CuO/SBA-16 prepared by different impregnation methods. Front. Chem. Eng. Chin 2008, 2, 150–154. [Google Scholar]
- Zhu, Y; Dong, Y; Zhao, L; Yuan, F. Preparation and characterization of mesoporous VOx/SBA-16 and their application for the direct catalytic hydroxylation of benzene to phenol. J. Mol. Catal. A: Chem 2010, 315, 205–212. [Google Scholar]
- Elangovan, SP; Inoue, K; Yokoi, T; Okubo, T; Kojima, A; Ogura, M. Solid acid porous materials for the catalytic transformation of 1-adamantol. Catal. Today 2008, 131, 367–371. [Google Scholar]
- Tsoncheva, T; Rosenholm, J; Teixeira, CV; Dimitrov, M; Linden, M; Minchev, C. Preparation, characterization and catalytic behavior in methanol decomposition of nanosized iron oxide particles within large pore ordered mesoporous silicas. Microporous Mesoporous Mater 2006, 89, 209–218. [Google Scholar]
- Wang, YQ; Yang, CM; Zibrowius, B; Spliethoff, B; Lindén, M; Schüth, F. Directing the formation of vinyl-functionalized silica to the hexagonal SBA-15 or large-pore Ia3d structure. Chem. Mater 2003, 15, 5029–5035. [Google Scholar]
- Leisant, C; Frébault, F; Delacôte, C; Lebeau, B; Marichal, C; Walcarius, A; Patarin, J. Synthesis and characterization of mesoporous silicas functionalized by thiol groups, and application as sorbents for mercury (II). Stud. Surf. Sci. Catal 2005, 156, 925–932. [Google Scholar]
- Grudzien, RM; Pikus, S; Jaroniec, M. Periodic mesoporous organosilicas with Im3m symmetry and large isocyanurate bridging groups. J. Phys. Chem. B: Lett 2006, 110, 2972–2975. [Google Scholar]
- Grudzien, RM; Blitz, JP; Pikus, S; Jaroniec, M. Cage-like mesoporous organosilicas with isocyanurate bridging groups: Synthesis, template removal and structural properties. Microporous Mesoporous Mater 2009, 118, 68–77. [Google Scholar]
- Grudzien, RM; Grabicka, BE; Knobloch, DJ; Jaroniec, M. Co-condensation synthesis and adsorption properties of cage-like mesoporous silicas with imidazole groups. Colloid Surface A: Physicochem. Eng. Aspect 2006, 291, 139–147. [Google Scholar]
- Wei, J; Shi, J; Pan, H; Su, Q; Zhu, J; Shi, Y. Thermal and hydrothermal stability of amino functionalized SBA-16 and promotion of hydrophobicity by silylation. Microporous Mesoporous Mater 2009, 117, 596–602. [Google Scholar]
- Knöfel, C; Descarpentries, J; Benzaouia, A; Zelenák, V; Mornet, S; Llewellyn, PL; Hornebecq, V. Functionalised micro-/mesoporous silica for the adsorption of carbon dioxide. Microporous Mesoporous Mater 2007, 99, 79–85. [Google Scholar]
- Han, S-C; Sujandi, E; Park, SE. Functionalization of SBA-16 mesoporous materials with cobalt (III) cage amine complex. Bull. Kor. Chem. Soc 2005, 26, 1381–1384. [Google Scholar]
- Ueno, Y; Ajito, K. Terahertz time-domain spectra of aromatic carboxylic acids incorporated in nano-sized pores of mesoporous silicate. Anal. Sci 2007, 23, 803–807. [Google Scholar]
- Sujandi, E; Prasetyanto, EA; Lee, S-C; Park, S-E. Microwave synthesis of large pored chloropropyl functionalized mesoporous silica with p6mm, Ia-3d, and Im3m structures. Microporous Mesoporous Mater 2009, 118, 134–142. [Google Scholar]
- Lee, S-C; Han, D-S; Jiang, N; Park, S-E. Self pH-Controlled Al-incorporation for Mesoporous Aluminosilicate as Lewis Acid Catalys; ACS Division of Petroleum Chemistry, Inc: New York, NY, USA, 2008; Volume 53, pp. 187–189. [Google Scholar]
- Shen, S; Deng, Y; Zhu, G; Mao, D; Wang, Y; Wu, G; Li, J; Liu, X; Lu, G; Zhao, D. Synthesis and characterization of Ti-SBA-16 ordered mesoporous silica composite. J. Mater. Sci 2007, 42, 7057–7061. [Google Scholar]
- Shah, AT; Li, B; Abdalla, ZEA. Direct synthesis of Ti-containing SBA-16-type mesoporous material by the evaporation-induced self assembly method and its catalytic performance for oxidative desulfurization. J. Colloid Interface. Sci 2009, 336, 707–711. [Google Scholar]
- Jermy, BR; Kim, S-Y; Bineesh, KV; Park, D-W. Optimization, synthesis and characterization of vanadium-substituted thick-walled three-dimensional SBA-16. Microporous Mesoporous Mater 2009, 117, 661–669. [Google Scholar]
- Jermy, BR; Kim, S-Y; Bineesh, KV; Selvaraj, M; Park, D-W. Easy route for the synthesis of Fe-SBA-16 at weak acidity and its catalytic activity in the oxidation of cyclohexene. Microporous Mesoporous Mater 2009, 121, 103–113. [Google Scholar]
- Feliczak-Guzik, A; Wawrzynczak, A; Nowak, I. Studies on mesoporous niobosilicates synthesized using F127 triblock copolymer. Adsorption 2009, 15, 247–253. [Google Scholar]
- Glomm, WR; Øye, G; Walmsley, J; Sjoblom, J. Synthesis and characterization of gold nanoparticle-functionalized ordered mesoporous materials. J. Dispers. Sci. Technol 2005, 26, 729–744. [Google Scholar]
- Lee, B; Ma, Z; Zhang, Z; Park, C; Dai, S. Influences of synthesis conditions and mesoporous structures on the gold nanoparticles supported on mesoporous silica hosts. Microporous Mesoporous Mater 2009, 122, 160–167. [Google Scholar]
- Shi, K-Y; Xin, B-F; Chi, Y-J; Fu, H-G. Assembling porous Fe2O3 nanowire arrays by electrochemical deposition in mesoporous silica SBA-16 films. Acta Chim. Sinica 2004, 62, 1859–1861. [Google Scholar]
- Son, WJ; Choi, JS; Ahn, WS. Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materials. Microporous Mesoporous Mater 2008, 113, 31–40. [Google Scholar]
- Wei, J; Shi, L; Pan, H; Zhao, W; Ye, Q; Shi, Y. Adsorption of carbon dioxide on organically functionalized SBA-16. Microporous Mesoporous Mater 2008, 116, 394–399. [Google Scholar]
- Xue, X; Li, F. Removal of Cu(II) from aqueous solution by adsorption onto functionalized SBA-16 mesoporous silica. Microporous Mesoporous Mater 2008, 116, 116–122. [Google Scholar]
- Terrés, E; Montiel, M; Le Borgne, S; Torres, E. Immobilization of chloroperoxidase on mesoporous materials for the oxidation of 4,6-dimethyldibenzothiophene, a recalcitrant organic sulfur compound present in petroleum fractions. Biotechnol. Lett 2008, 30, 173–179. [Google Scholar]
- Shi, K; Peng, LM; Chen, Q; Wang, R; Zhou, W. Porous crystalline iron oxide thin films templated by mesoporous silica. Microporous Mesoporous Mater 2005, 83, 219–224. [Google Scholar]
- Guo, W; Su, F; Zhao, XS. Ordered mesostructured carbon templated by SBA-16 silica. Lett. Ed./Carbon 2005, 43, 2397–2429. [Google Scholar]
- Guo, W; Zhao, XS. Well-ordered cubic mesoporous carbon with Im3m symmetry. Stud. Surf. Sci. Catal 2005, 156, 551–556. [Google Scholar]
- Vinu, A; Srinivasu, P; Sawant, DP; Mori, T; Ariga, K; Chang, J-C; Jhung, SH; Balasubramanian, VV; Hwang, YK. Three-dimensional cage type mesoporous cn-based hybrid material with very high surface area and pore volume. Chem. Mater 2007, 19, 4367–4372. [Google Scholar]
- Yue, W; Hill, AH; Harrison, A; Zhou, W. Mesoporous single-crystal Co3O4 templated by cage-containing mesoporous silica. Chem. Commun 2007, 2518–2520. [Google Scholar]
- Shon, JK; Kong, SS; Kim, YS; Lee, J-H; Park, WK; Park, SC; Kim, JM. Solvent-free infiltration method for mesoporous SnO2 using mesoporous silica templates. Microporous Mesoporous Mater 2009, 120, 441–446. [Google Scholar]
- Su, H; Jing, L; Shi, K; Yao, C; Fu, H. Synthesis of large surface area LaFeO3 nanoparticles by SBA-16 template method as high active visible photocatalysts. J. Nanopart Res 2009. [Google Scholar] [CrossRef]
- Mohanty, P; Landskron, K. Synthesis of periodic mesoporous phosphorus–nitrogen frameworks by nanocasting from mesoporous silica using melt-infiltration. J. Mater. Chem 2009, 19, 2400–2406. [Google Scholar]
- Yamada, T; Zhou, HS; Uchida, H; Tomita, M; Ueno, Y; Honma, I; Asai, K; Katsube, T. Application of a cubic-like mesoporous silica film to a surface photovoltage gas sensing system. Microporous Mesoporous Mater 2002, 54, 269–276. [Google Scholar]
- Yamada, T; Zhou, HS; Uchida, H; Honma, I; Katsube, T. Experimental and Theoretical NOx Physisorption Analyses of Mesoporous Film (SBA-15 and SBA-16) Constructed Surface Photo Voltage (SPV) Sensor. J. Phys. Chem. B 2004, 108, 13341–13346. [Google Scholar]
- Ueno, Y; Tate, A; Niwa, O; Zhou, HS; Yamada, T; Honma, I. High benzene selectivity of mesoporous silicate for BTX gas sensing microfluidic devices. Anal. Bioanal. Chem 2005, 382, 804–809. [Google Scholar]
- Fuertes, AB; Lota, G; Centeno, TA; Frackowiak, E. Templated mesoporous carbons for supercapacitor application. Electrochim. Acta 2005, 50, 2799–2805. [Google Scholar]
- Lin, JJ; Wang, XD. Novel low-κ polyimide/mesoporous silica composite films: Preparation, microstructure, and properties. Polymer 2007, 48, 318–329. [Google Scholar]
- Lin, JJ; Wang, XD. Preparation, microstructure, and properties of novel low-κ brominated epoxy/mesoporous silica composites. Eur. Polym. J 2008, 44, 1414–1427. [Google Scholar]
- Lin, JJ; Wang, XD. New type of low-dielectric composites based on o-cresol novolac epoxy resin and mesoporous silicas: Fabrication and performances. J. Mater. Sci 2008, 43, 4455–4465. [Google Scholar]
- Marschall, R; Bannat, I; Caro, J; Wark, M. Proton conductivity of sulfonic acid functionalised mesoporous materials. Microporous Mesoporous Mater 2007, 99, 190–196. [Google Scholar]
- Manning, J; Campbell, R; Bakker, MG; Li, X; Lee, DR; Wang, J. Development of 3-D magnetic nano-arrays by electrodeposition into mesoporous silica films. Mater. Res. Soc. Symp. Proc 2006, 961, 126–131. [Google Scholar]
- Tu, J; Wang, R; Geng, W; Lai, X; Zhang, T; Li, N; Yue, N; Li, X. Humidity sensitive property of Li-doped 3D periodic mesoporous silica SBA-16. Sens. Actuat. B 2009, 136, 392–398. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rivera-Muñoz, E.M.; Huirache-Acuña, R. Sol Gel-Derived SBA-16 Mesoporous Material. Int. J. Mol. Sci. 2010, 11, 3069-3086. https://doi.org/10.3390/ijms11093069
Rivera-Muñoz EM, Huirache-Acuña R. Sol Gel-Derived SBA-16 Mesoporous Material. International Journal of Molecular Sciences. 2010; 11(9):3069-3086. https://doi.org/10.3390/ijms11093069
Chicago/Turabian StyleRivera-Muñoz, Eric M., and Rafael Huirache-Acuña. 2010. "Sol Gel-Derived SBA-16 Mesoporous Material" International Journal of Molecular Sciences 11, no. 9: 3069-3086. https://doi.org/10.3390/ijms11093069