MicroRNAs miR-27a and miR-143 Regulate Porcine Adipocyte Lipid Metabolism
Abstract
:1. Introduction
2. Results and Discussion
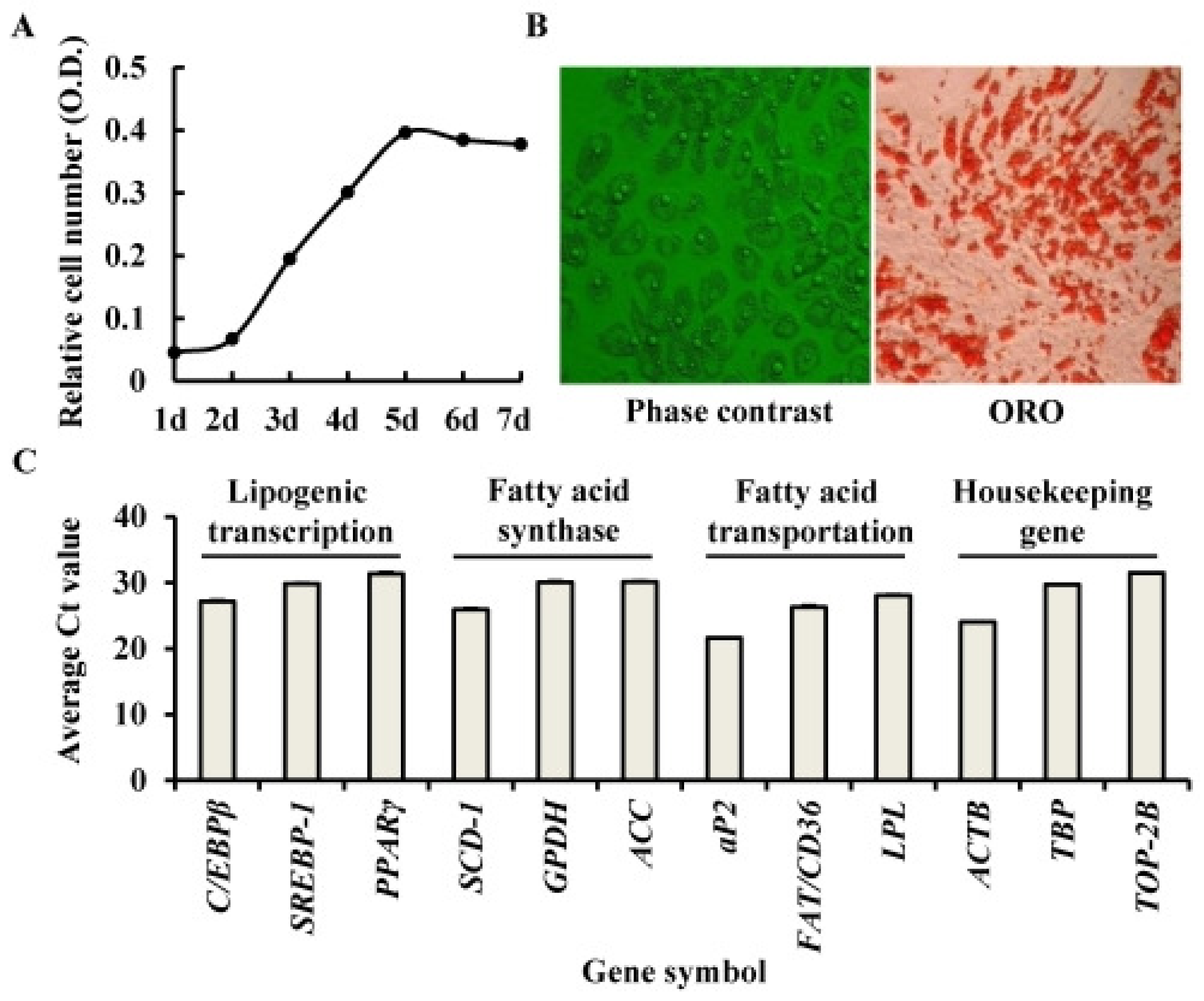
2.1. Identification of Porcine Adipocyte
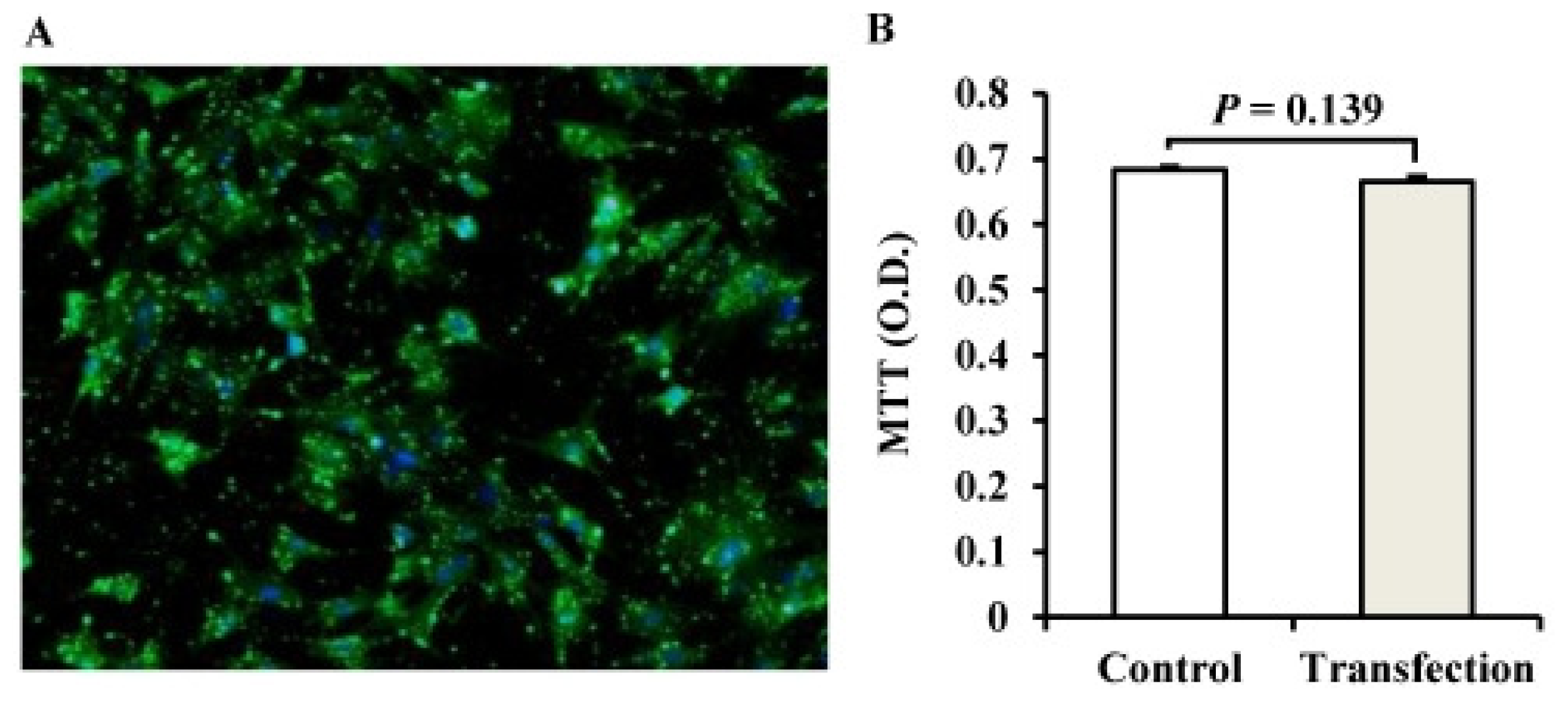
2.2. The Establishment of a miRNA Transfection System for Porcine Adipocytes
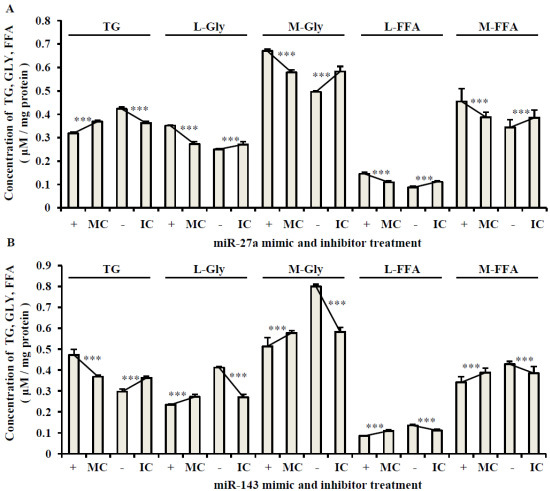
2.3. The Roles of miR-27a and miR-143 in Porcine Adipocyte Lipid Metabolism
3. Experimental Section
3.1. Primary Culture of Porcine S-V Cells
3.2. Differentiation of Adipocytes
3.3. Transient Transfection with the Synthetic miRNA Mimics and Inhibitors
3.4. Lipid Metabolism Assay
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Bagga, S.; Bracht, J.; Hunter, S.; Massirer, K.; Holtz, J.; Eachus, R.; Pasquinelli, A.E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 2005, 122, 553–563. [Google Scholar]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–858. [Google Scholar]
- Xu, P.; Vernooy, S.Y.; Guo, M.; Hay, B.A. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr. Biol 2003, 13, 790–795. [Google Scholar]
- Poy, M.N.; Eliasson, L.; Krutzfeldt, J.; Kuwajima, S.; Ma, X.; Macdonald, P.E.; Pfeffer, S.; Tuschl, T.; Rajewsky, N.; Rorsman, P.; et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004, 432, 226–230. [Google Scholar]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 2006, 3, 87–98. [Google Scholar]
- Rayner, K.J.; Suárez, Y.; Dávalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernández-Hernando, C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 2010, 328, 1570–1573. [Google Scholar]
- Kim, S.Y.; Kim, A.Y.; Lee, H.W.; Son, Y.H.; Lee, G.Y.; Lee, J.W.; Lee, Y.S.; Kim, J.B. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPAR [gamma] expression. Biochem. Biophys. Res. Commun 2010, 392, 323–328. [Google Scholar]
- Esau, C.; Kang, X.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 regulates adipocyte differentiation. J. Biol. Chem 2004, 279, 52361–52365. [Google Scholar]
- Kajimoto, K.; Naraba, H.; Iwai, N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA 2006, 12, 1626–1632. [Google Scholar]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J 2008, 29, 2959–2971. [Google Scholar]
- Alexander, R.; Lodish, H.; Sun, L. MicroRNAs in adipogenesis and as therapeutic targets for obesity. Expert Opin. Ther. Targets 2011, 15, 623–636. [Google Scholar]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011, 474, 649–653. [Google Scholar]
- Sebert, S.P.; Lecannu, G.; Kozlowski, F.; Siliart, B.; Bard, J.M.; Krempf, M.; Champ, M.M. Childhood obesity and insulin resistance in a Yucatan mini-piglet model: Putative roles of IGF-1 and muscle PPARs in adipose tissue activity and development. Int. J. Obes. (Lond. ) 2005, 29, 324–333. [Google Scholar]
- Du, Z.Q.; Fan, B.; Zhao, X.; Amoako, R.; Rothschild, M.F. Association analyses between type 2 diabetes genes and obesity traits in pigs. Obesity 2008, 17, 323–329. [Google Scholar]
- Takanabe, R.; Ono, K.; Abe, Y.; Takaya, T.; Horie, T.; Wada, H.; Kita, T.; Satoh, N.; Shimatsu, A.; Hasegawa, K. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem. Biophys. Res. Commun 2008, 376, 728–732. [Google Scholar]
- Bai, L.; Pang, W.J.; Yang, Y.J.; Yang, G.S. Modulation of Sirt1 by resveratrol and nicotinamide alters proliferation and differentiation of pig preadipocytes. Mol. Cell. Biochem 2008, 307, 129–140. [Google Scholar]
- Li, B.; Zerby, H.; Lee, K. Heart fatty acid binding protein is upregulated during porcine adipocyte development. J. Anim. Sci 2007, 85, 1651–1659. [Google Scholar]
- Hauner, H.; Entenmann, G.; Wabitsch, M.; Gaillard, D.; Ailhaud, G.; Negrel, R.; Pfeiffer, E.F. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J. Clin. Invest 1989, 84, 1663–1670. [Google Scholar]
- Meyer-Rochow, G.Y.; Jackson, N.E.; Conaglen, J.V.; Whittle, D.E.; Kunnimalaiyaan, M.; Chen, H.; Westin, G.; Sandgren, J.; Stalberg, P.; Khanafshar, E.; et al. MicroRNA profiling of benign and malignant pheochromocytomas identifies novel diagnostic and therapeutic targets. Endocr. Relat. Cancer 2010, 17, 835–846. [Google Scholar]



| Gene Symbol | GenBank ID | Primer Sequence (5′→3′) | Product Size (bp) | |
|---|---|---|---|---|
| Lipogenic Transcription | C/EBP γ | DQ450678 | F: GTCCAAACCAACCGCACAT R: GAAACAACCCCGTAGGAACAT | 262 |
| PPAR γ | AB097926 | F: GTTGATTTCTCCAGCATTTCCA R: GGCTCTTCGTGAGGTTTGTTG | 188 | |
| SREBP-1 | AY496867 | F: AAGCGGACGGCTCACAATG R: CGCAAGACGGCGGATTTAT | 122 | |
| Fatty Acid Synthase | GPDH | U97255 | F: TGTGACTGGAAAACGGTGGC R: CGTGCAGGCATACTCCTTAATT | 103 |
| ACC | AF175308 | F: AAAGAGGTTCCAGGCACAGTC R: CGGTGGGAGGTATGCTTGAGT | 118 | |
| SCD-1 | AY487829 | F: GAATGACGTTTATGAATGGGC R: CAGCTTCTCGGCTTTTAGGT | 190 | |
| Fatty Acid Transportation | LPL | NM_214286 | F: TGCCCTGTAACTTCTACCCCA R: GGCAAGTGTCCTCAACTGTGTC | 208 |
| FAT/CD36 | DQ192230 | F: GGGTTAAAACAGGCACGGAA R: TGGCACCATTGGGCTGTAGG | 201 | |
| aP2 | DQ450677 | F: GACAGGAAAGTCAAGAGCACCA R: CGGGACAATACATCCAACAGAGT | 228 | |
| Housekeeping Gene | ACTB * | DQ178122 | F: TCTGGCACCACACCTTCT R: TGATCTGGGTCATCTTCTCAC | 114 |
| TBP * | DQ178129 | F: GATGGACGTTCGGTTTAGG R: AGCAGCACAGTACGAGCAA | 124 | |
| TOP2B * | AF222921 | F: AACTGGATGATGCTAATGATGCT R: TGGAAAAACTCCGTATCTGTCTC | 137 | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, T.; Li, M.; Guan, J.; Li, P.; Wang, H.; Guo, Y.; Shuai, S.; Li, X. MicroRNAs miR-27a and miR-143 Regulate Porcine Adipocyte Lipid Metabolism. Int. J. Mol. Sci. 2011, 12, 7950-7959. https://doi.org/10.3390/ijms12117950
Wang T, Li M, Guan J, Li P, Wang H, Guo Y, Shuai S, Li X. MicroRNAs miR-27a and miR-143 Regulate Porcine Adipocyte Lipid Metabolism. International Journal of Molecular Sciences. 2011; 12(11):7950-7959. https://doi.org/10.3390/ijms12117950
Chicago/Turabian StyleWang, Tao, Mingzhou Li, Jiuqiang Guan, Penghao Li, Huiyu Wang, Yanqin Guo, Surong Shuai, and Xuewei Li. 2011. "MicroRNAs miR-27a and miR-143 Regulate Porcine Adipocyte Lipid Metabolism" International Journal of Molecular Sciences 12, no. 11: 7950-7959. https://doi.org/10.3390/ijms12117950