A Mitochondrial Membrane Exopolyphosphatase Is Modulated by, and Plays a Role in, the Energy Metabolism of Hard Tick Rhipicephalus (Boophilus) microplus Embryos
Abstract
:1. Introduction
2. Results and Discussion
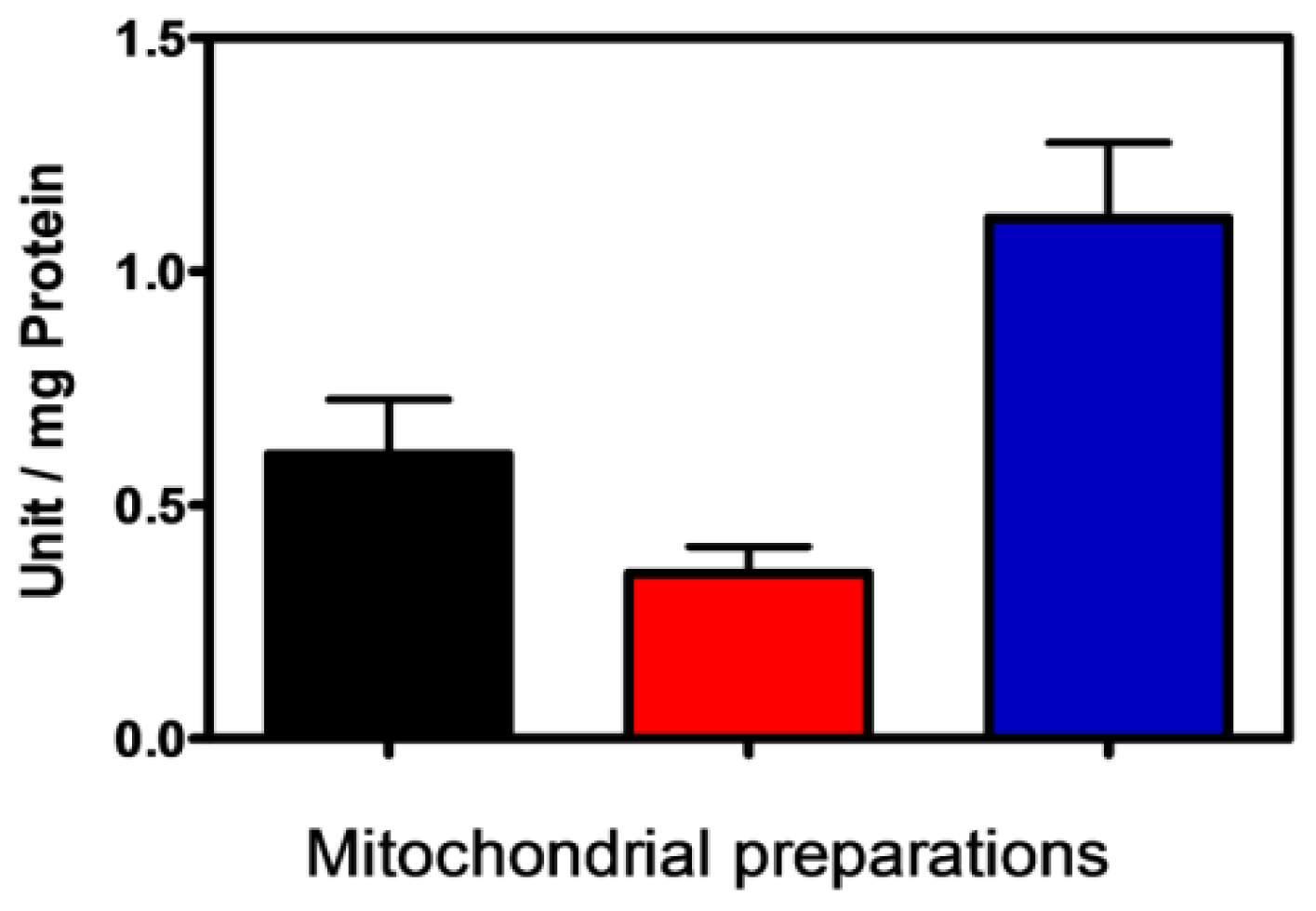
2.1. Characterization of Isolated Mitochondria
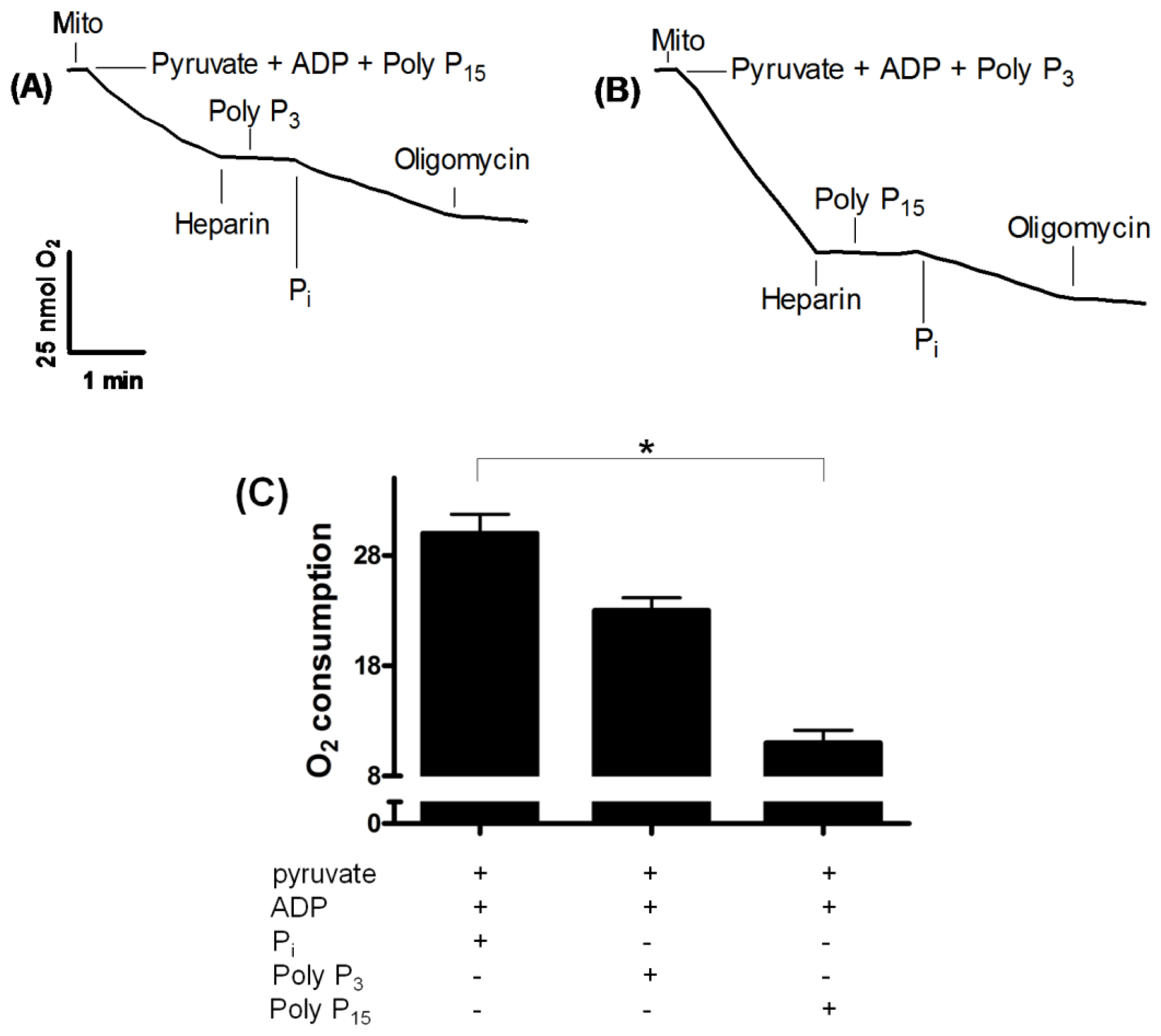
2.2. Influence of Membrane PPX in Mitochondrial Respiration
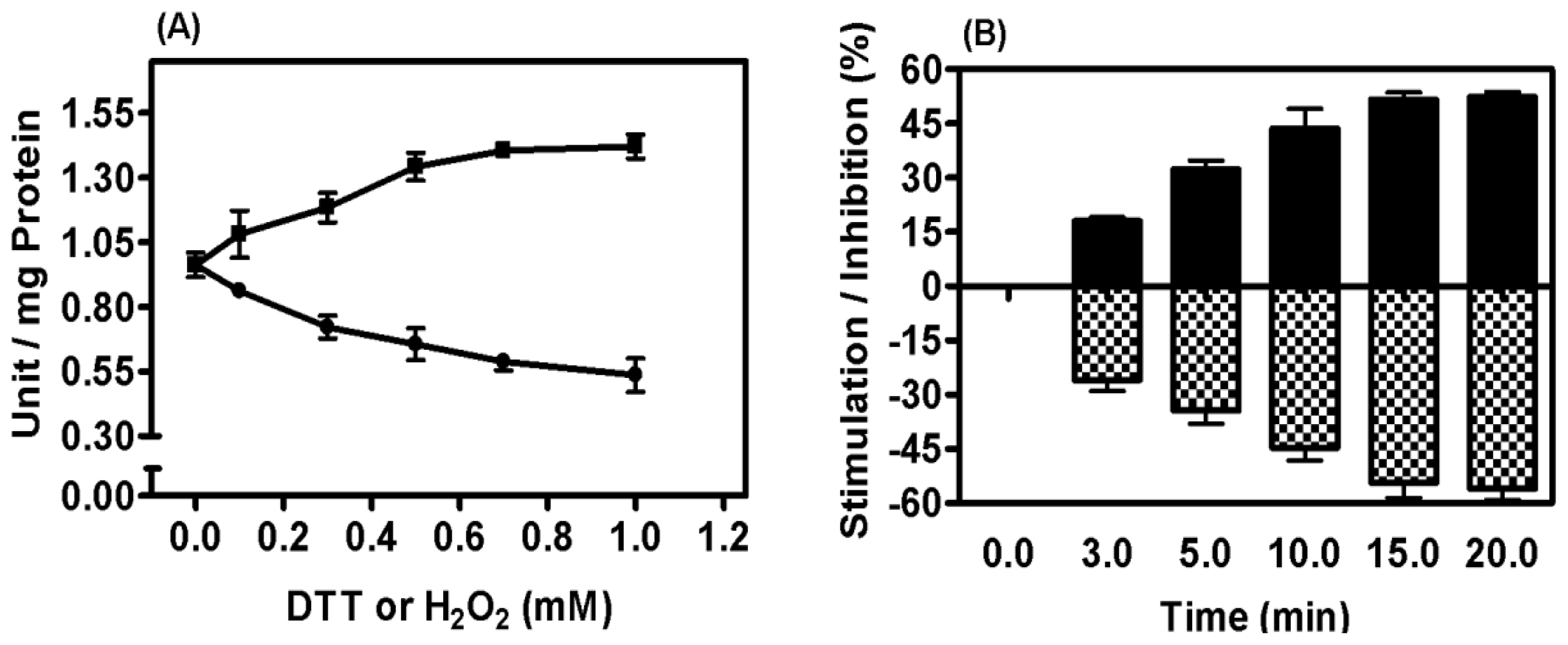
2.3. Characterization of PPX Activity in Mitochondrial Membrane Preparation
2.4. PPX Activity during Mitochondrial Respiration
2.5. Mitochondrial Membrane PPX Redox Sensitivity
3. Experimental Section
3.1. Ticks
3.2. Chemicals Materials
3.3. Isolation of Mitochondria
3.4. PPX Assay and Kinetic Parameters
3.5. Respiration Measurements
4. Conclusion
Acknowledgments
References
- Kornberg, A; Rao, NN; Ult-Riche, D. Inorganic polyphosphate: A molecule of many functions. Annu. Rev. Biochem 1999, 68, 89–125. [Google Scholar]
- Kulaev, I; Kulakovskaya, T. Polyphosphate and phosphate pump. Annu. Rev. Microbiol 2000, 54, 709–734. [Google Scholar]
- Reusch, RN. Poly-beta-hydroxybutyrate/calcium polyphosphate complexes in eukaryotic membranes. Proc. Soc. Exp. Biol. Med 1989, 191, 377–381. [Google Scholar]
- Jones, HE; Holland, IB; Jacq, IB; Wall, T; Campbell, AK. Escherichia coli lacking the AcrAB multidrug efflux pump also lacks nonproteinaceous, PHB–polyphosphate Ca2+ channels in the membrane. Biochim. Biophys. Acta 2003, 1612, 90–97. [Google Scholar]
- Kim, KS; Rao, NN; Fraley, CD; Kornberg, A. Inorganic polyphosphate is essential for long-term survival and virulence factors in Shigella and Salmonella spp. Proc. Natl. Acad. Sci. USA 2002, 99, 7675–7680. [Google Scholar]
- Rashid, MH; Rumbaugh, K; Passador, L; Davies, DG; Hamood, AN; Iglewski, BH; Kornberg, A. Polyphosphate kinase is essential for biofilm development, quorum sensing, and virulence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2000, 97, 9636–9641. [Google Scholar]
- McInerney, P; Mizutani, T; Shiba, T. Inorganic polyphosphate interacts with ribosomes and promotes translation fidelity in vitro and in vivo. Mol. Microbiol 2006, 60, 438–447. [Google Scholar]
- Kuroda, A; Nomura, K; Ohtomo, R; Kato, J; Ikeda, T; Takiguchi, N; Ohtake, H; Kornberg, A. Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 2001, 293, 705–708. [Google Scholar]
- Negoda, A; Negoda, E; Xian, M; Reusch, RN. Role of polyphosphate in regulation of the Streptomyces lividans KcsA channel. Biochim. Biophys. Acta 2009, 1788, 608–614. [Google Scholar]
- Wang, L; Fraley, CD; Faridi, J; Kornberg, A; Roth, RA. Inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11249–11254. [Google Scholar]
- Smith, SA; Mutch, NJ; Baskar, D; Rohloff, P; Docampo, R; Morrissey, JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 903–908. [Google Scholar]
- Lorenz, B; Munkner, J; Oliveira, MP; Kuusksalu, A; Leitao, JM; Muller, WE; Schroder, HC. Changes in metabolism of inorganic polyphosphate in rat tissues and human cells during development and apoptosis. Biochim. Biophys. Acta 1997, 1335, 51–60. [Google Scholar]
- Hernandez-Ruiz, L; Gonzalez-Garcia, I; Castro, C; Brieva, JA; Ruiz, FA. Inorganic polyphosphate and specific induction of apoptosis in human plasma cells. Haematologica 2006, 91, 1180–1186. [Google Scholar]
- Kawano, MM. Inorganic polyphosphate induces apoptosis specifically in human plasma cells. Haematologica 2006, 91, 1154A. [Google Scholar]
- Campos, E; Facanha, A; Moraes, J; da Silva, VI; Masuda, A; Logullo, C. A mitochondrial exopolyphosphatase activity modulated by phosphate demand in Rhipicephalus (Boophilus) microplus embryo. Insect Biochem. Mol. Biol 2007, 37, 1103–1107. [Google Scholar]
- Campos, E; Facanha, A; Pessoa, EP; da Silva, VI; Masuda, A; Logullo, C. Exopolyphosphatases in nuclear and mitochondrial fractions during the hard tick Rhipicephalus (Boophilus) microplus embryo. Comp. Biochem. Physiol 2008, 151, 311–316. [Google Scholar]
- Kumble, KD; Kornberg, A. Endopolyphosphatases for long chain inorganic polyphosphate in yeast and mammals. J. Biol. Chem 1996, 271, 27146–27151. [Google Scholar]
- Huh, WK; Falvo, JV; Gerke, LC; Carroll, AS; Howson, RW; Weissman, JS; O’Shea, EK. Global analysis of protein localization in budding yeast. Nature 2003, 425, 686–691. [Google Scholar]
- Pestov, NA; Kulakovskaya, TV; Kulaev, IS. Effects of inactivation of the PPN1 gene on exopolyphosphatases, inorganic polyphosphates and function of mitochondria in the yeast Saccharomyces cerevisiae. FEMS Yeast Res 2005, 5, 823–828. [Google Scholar]
- Beauvoit, B; Rigoulet, M; Guerin, B; Canioni, P. Polyphosphates as a source of high-energy phosphates in yeast mitochondria: A 31P NMR-study. FEBS Lett 1989, 252, 17–21. [Google Scholar]
- Guerrero, FD; Nene, VM; George, JE; Barker, SC; Willadsen, P. Sequencing a new target genome: The Boophilus microplus (Acari: Ixodidae) genome project. J. Med. Entomol 2006, 43, 9–16. [Google Scholar]
- Sonenshine, DE; Kocan, KM; de La, FJ. Tick control: Further thoughts on a research agenda. Trends Parasitol 2006, 22, 550–551. [Google Scholar]
- Fagotto, F. Yolk degradation in tick eggs: I. Occurrence of a cathepsin L-like acid proteinase in yolk spheres. Arch. Insect Biochem. Physiol 1990, 14, 217–235. [Google Scholar]
- Gabel, NM; Thomas, V. Evidence for the occurrence and distribution of inorganic polyphosphates in vertebrate tissues. J. Neurochem 1971, 18, 1229–1242. [Google Scholar]
- Pavlov, E; Zakharian, E; Bladen, C; Diao, CTM; Grimbly, C; Reusch, RN; French, RJ. A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys. J 2005, 88, 2614–2625. [Google Scholar]
- Gomes, FM; Oliveira, DMP; Motta, LM; Ramos, IB; Miranda, K; Machado, EA. Inorganic polyphosphate inhibits an aspartic protease–like activity in the eggs of Rhodnius prolixus (Stahl) and impairs yolk mobilization in vitro. J. Cell. Physiol 2010, 222, 606–611. [Google Scholar]
- Bate, M; Arias, AM. The embryonic origin of imaginal discs in Drosophila. Development 1991, 112, 755–761. [Google Scholar]
- Monnerat, AT; Machado, MP; Vale, BS; Soares, MJ; Lima, JB; Lenzi, HL; Valle, D. Anopheles albitarsis embryogenesis: Morphological identification of major events. Mem. Inst. Oswaldo Cruz 2002, 97, 589–596. [Google Scholar]
- Campos, E; Moraes, J; Facanha, AR; Moreira, E; Valle, D; Abreu, L; Manso, PP; Nascimento, A; Pelajo-Machado, M; Lenzi, H; Masuda, A; Vaz, IS; Logullo, C. Kinetics of energy source utilization in Boophilus microplus (Canestrini, 1887) (Acari: Ixodidae) embryonic development. Vet. Parasitol 2006, 138, 349–357. [Google Scholar]
- Rodrigues, CO; Ruiz, FA; Vieira, M; Hill, JE; Docampo, R. An acidocalcisomal exopolyphosphatase from Leishmania major with high affinity for short chain polyphosphate. J. Biol. Chem 2002, 277, 50899–50906. [Google Scholar]
- Lichko, LP; Kulakovskaya, TV; Kulaev, IS. Membrane-bound and soluble polyphosphatases of mitochondria of Saccharomyces cerevisiae: identification and comparative characterization. Biochim. Biophys. Acta 1998, 1372, 153–162. [Google Scholar]
- Pavlov, E; Aschar-Sobbi, R; Campanella, M; Turner, RJ; Gómes-García, M; Abramov, AY. Inorganic polyphosphate and energy metabolism in mammalian cells. J. Biol. Chem 2010, 285, 9420–9428. [Google Scholar]
- Abramov, AY; Fraley, C; Diao, CT; Winkfein, R; Colicos, MA; Duchen, MR; French, RJ; Pavlov, E. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc. Natl. Acad. Sci. USA 2007, 104, 18091–18096. [Google Scholar]
- Reusch, NM; Sadoff, HL. Putative structure and functions of a poly-beta-hydroxybutyrate/ calcium polyphosphate channel in bacterial plasma membranes. Proc. Natl. Acad. Sci. USA 1988, 85, 4176–4180. [Google Scholar]
- Fiske, CF; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem 1925, 66, 375–400. [Google Scholar]
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]

| State 3 | State 3 | RCR | |
|---|---|---|---|
| Mitochondria | 30.2 ± 3.2 | 4.6 ± 0.7 | 6.5 ± 0.4 |
| Substrates | Km (μM) | Vmax (μmol·min−1·mg protein−1) | Heparin (% inhibition) | O2 consumption (nmol.min−1·mg protein−1) |
|---|---|---|---|---|
| PolyP3 | 0.2 | 2.4 | 98 | 23.85 ± 2.06 |
| PolyP15 | 2.2 | 1.1 | 98 | 11.44 ± 1.79 |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Campos, E.; Façanha, A.R.; Costa, E.P.; Fraga, A.; Moraes, J.; Da Silva Vaz Jr., I.; Masuda, A.; Logullo, C. A Mitochondrial Membrane Exopolyphosphatase Is Modulated by, and Plays a Role in, the Energy Metabolism of Hard Tick Rhipicephalus (Boophilus) microplus Embryos. Int. J. Mol. Sci. 2011, 12, 3525-3535. https://doi.org/10.3390/ijms12063525
Campos E, Façanha AR, Costa EP, Fraga A, Moraes J, Da Silva Vaz Jr. I, Masuda A, Logullo C. A Mitochondrial Membrane Exopolyphosphatase Is Modulated by, and Plays a Role in, the Energy Metabolism of Hard Tick Rhipicephalus (Boophilus) microplus Embryos. International Journal of Molecular Sciences. 2011; 12(6):3525-3535. https://doi.org/10.3390/ijms12063525
Chicago/Turabian StyleCampos, Eldo, Arnoldo R. Façanha, Evenilton P. Costa, Amanda Fraga, Jorge Moraes, Itabajara Da Silva Vaz Jr., Aoi Masuda, and Carlos Logullo. 2011. "A Mitochondrial Membrane Exopolyphosphatase Is Modulated by, and Plays a Role in, the Energy Metabolism of Hard Tick Rhipicephalus (Boophilus) microplus Embryos" International Journal of Molecular Sciences 12, no. 6: 3525-3535. https://doi.org/10.3390/ijms12063525






