Recent Advances in Nanoparticle-Based Förster Resonance Energy Transfer for Biosensing, Molecular Imaging and Drug Release Profiling
Abstract
:1. Introduction
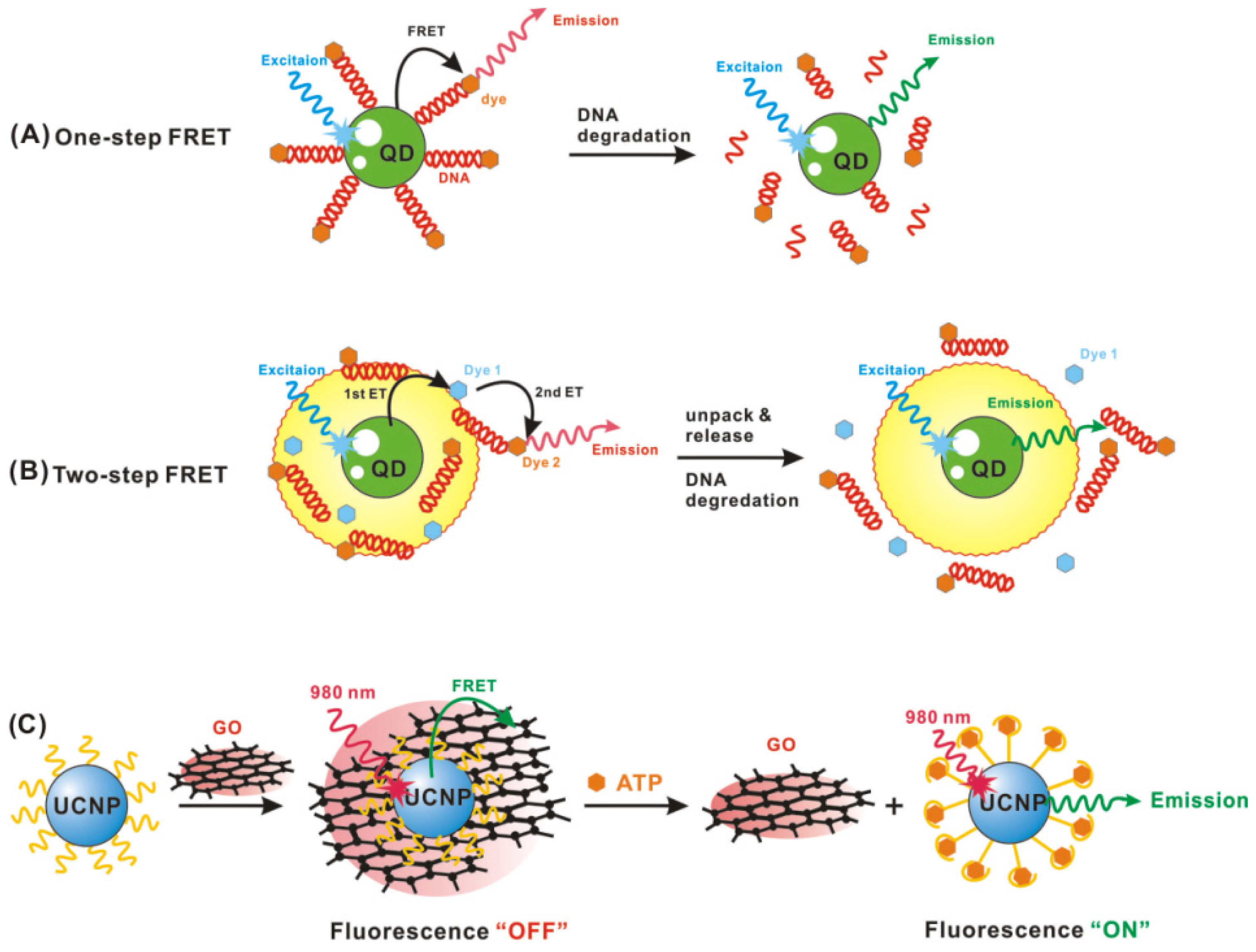
2. Applications in Biosensing
2.1. Quantum Dot-Based Biosensing
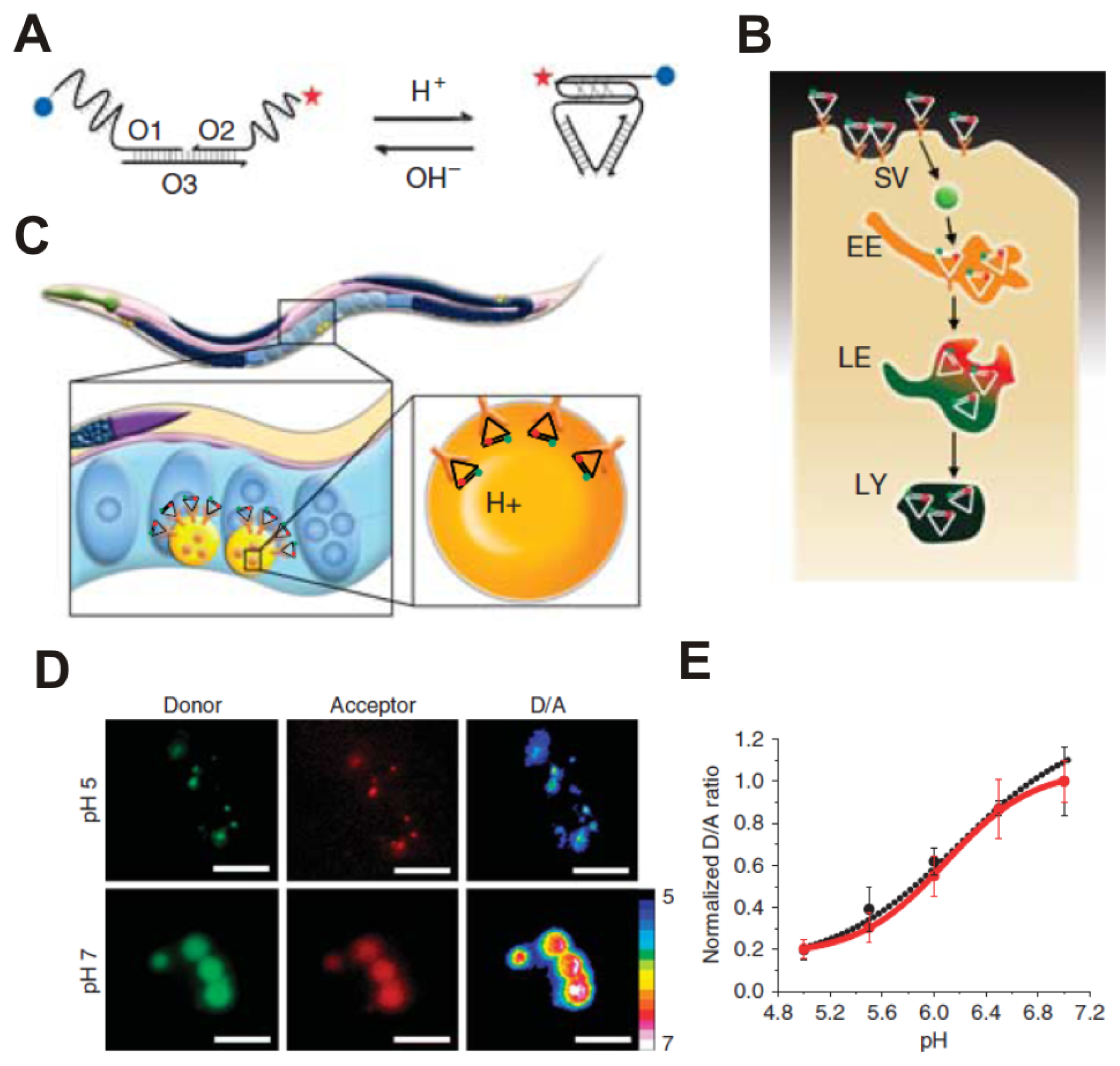
2.2. Rare-Earth-Doped Upconversion Nanoparticle-Based Biosensing
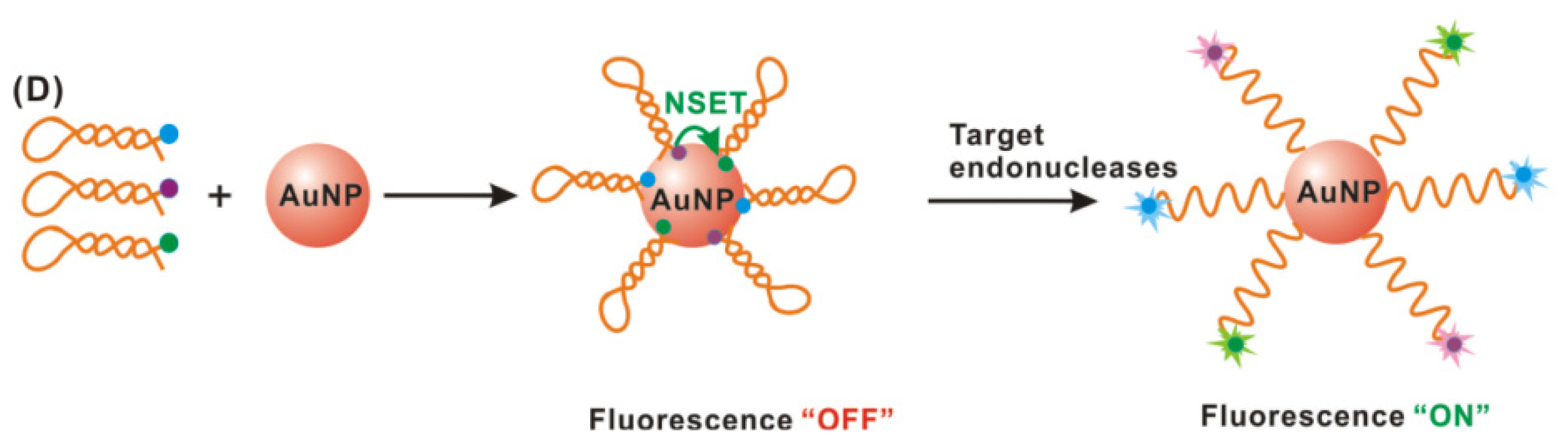
2.3. Gold Nanoparticle-Based Biosensing
3. Application of Molecular Imaging
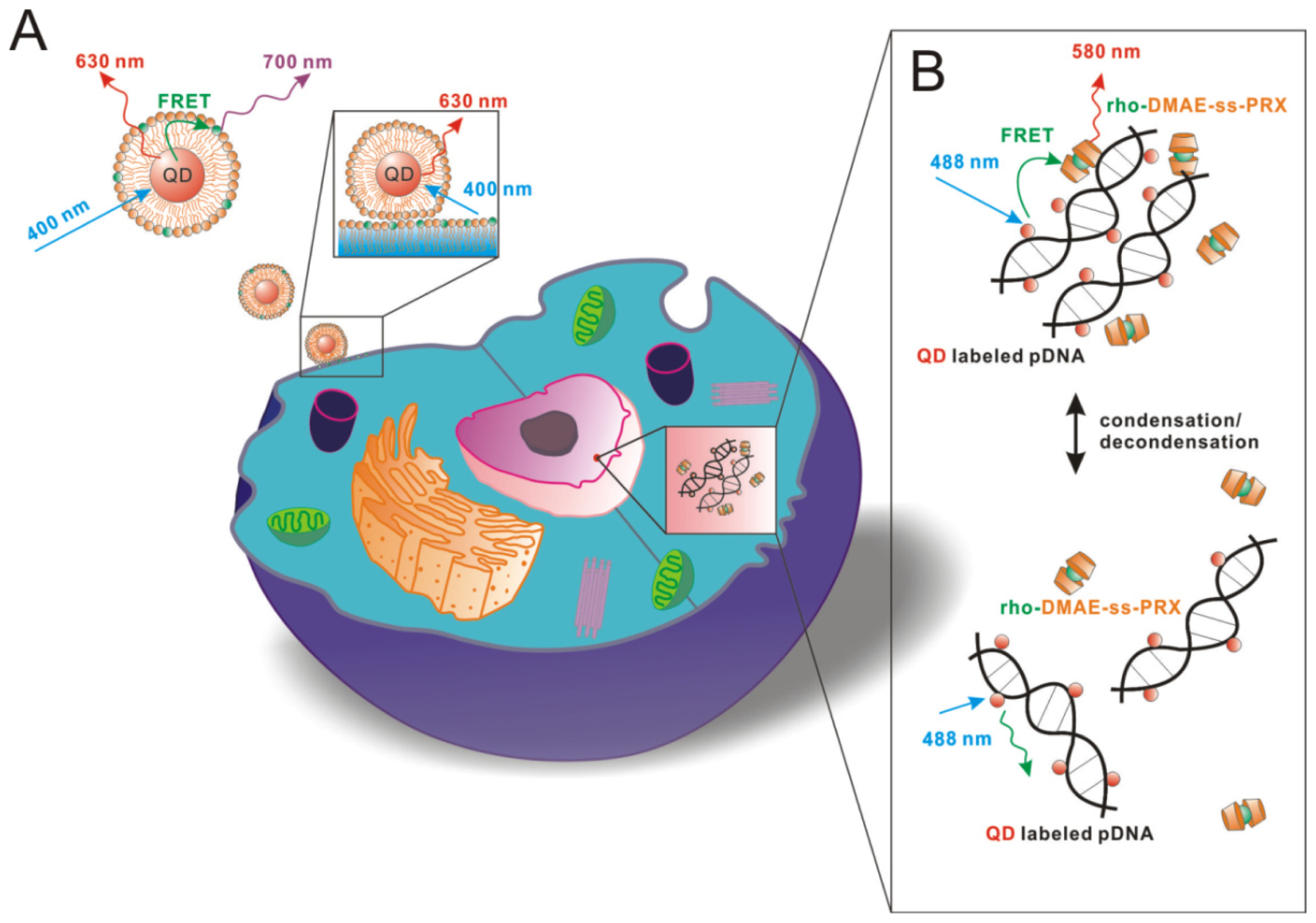
3.1. Quantum Dot-Based Molecular Imaging
3.2. Polymer-Based Molecular Imaging
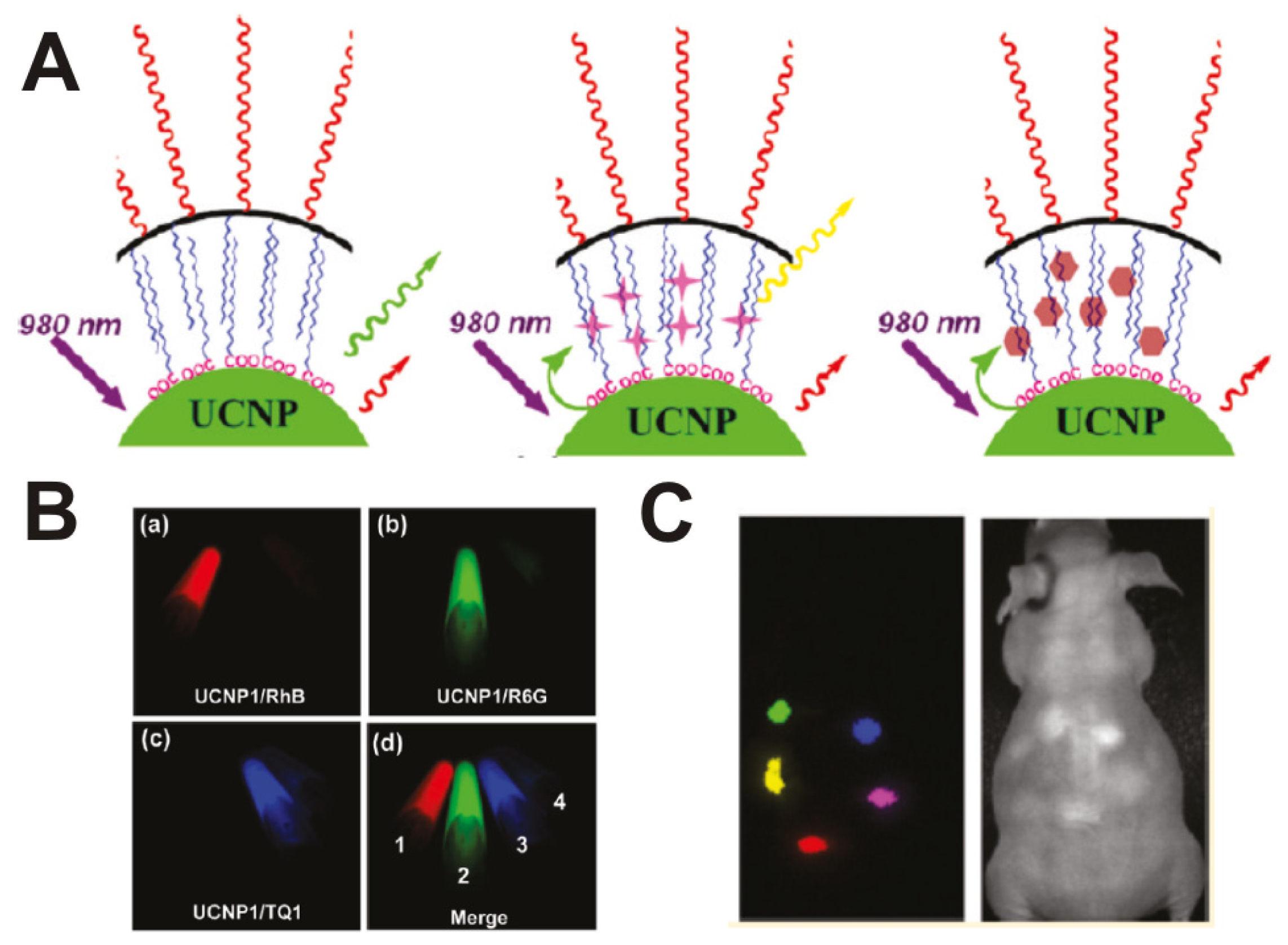
3.3. Rare-Earth-Doped Upconversion Nanoparticle-Based Molecular Imaging
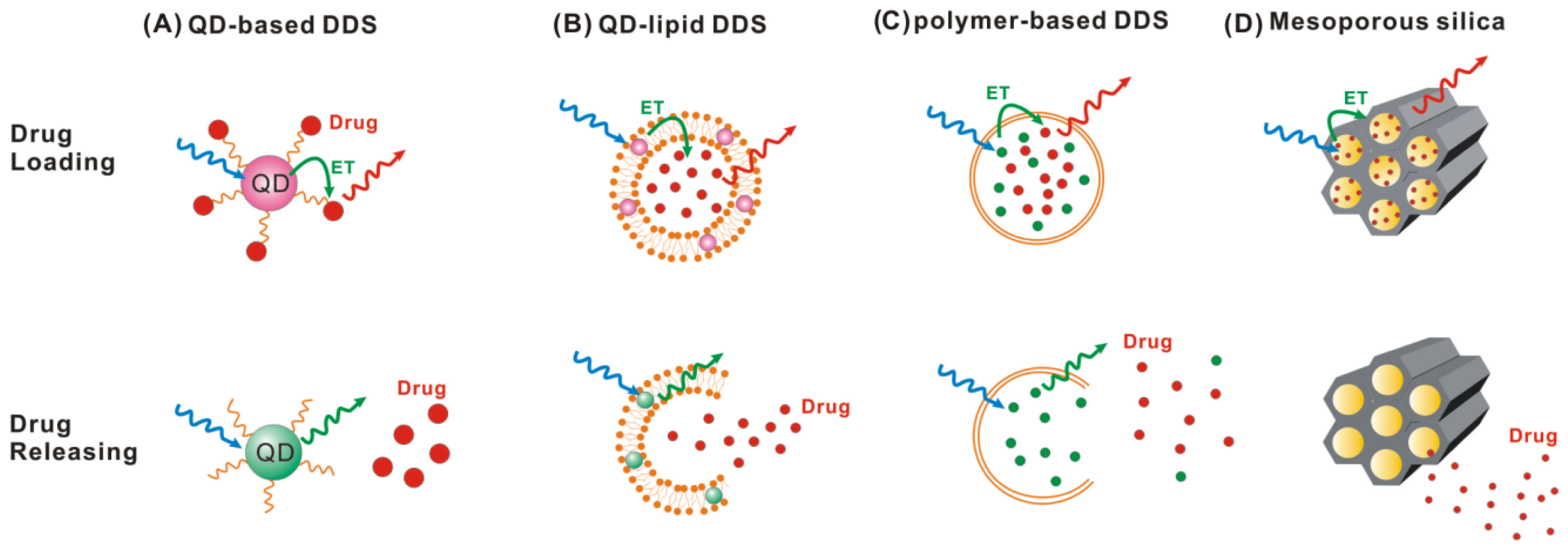
4. Application in Dynamic Monitoring Drug Releasing
4.1. Quantum Dot-Based Drug Delivery System
4.2. Polymer-Based Drug Delivery System
4.3. Mesoporous Silica Nanoparticle-Based Drug Delivery System
5. Conclusion
References
- Krukenberg, K.A.; Street, T.O.; Lavery, L.A.; Agard, D.A. Conformational dynamics of the molecular chaperone Hsp90. Q. Rev. Biophys 2011, 44, 229–255. [Google Scholar]
- Krusinski, T.; Ozyhar, A.; Dobryszycki, P. Dual FRET assay for detecting receptor protein interaction with DNA. Nucl. Acids Res 2010, 38, e108. [Google Scholar]
- Boeneman, K.; Mei, B.C.; Dennis, A.M.; Bao, G.; Deschamps, J.R.; Mattoussi, H.; Medintz, I.L. Sensing caspase 3 activity with quantum dot-fluorescent protein assemblies. J. Am. Chem. Soc 2009, 131, 3828–3829. [Google Scholar]
- Choi, Y.; Lee, J.; Kim, K.; Kim, H.; Sommer, P.; Song, R. Fluorogenic assay and live cell imaging of HIV-1 protease activity using acid-stable quantum dot-peptide complex. Chem. Commun 2010, 46, 9146–9148. [Google Scholar]
- Kim, Y.P.; Oh, Y.H.; Oh, E.; Ko, S.; Han, M.K.; Kim, H.S. Energy transfer-based multiplexed assay of proteases by using gold nanoparticle and quantum dot conjugates on a surface. Anal. Chem 2008, 80, 4634–4641. [Google Scholar]
- Kimura, R.H.; Steenblock, E.R.; Camarero, J.A. Development of a cell-based fluorescence resonance energy transfer reporter for Bacillus anthracis lethal factor protease. Anal. Biochem 2007, 369, 60–70. [Google Scholar]
- Kim, Y.P.; Oh, Y.H.; Oh, E.; Kim, H.-S. Chip-based protease assay using fluorescence resonance energy transfer between quantum dots and fluorophores. Biochip J 2007, 1, 228–233. [Google Scholar]
- Sahoo, H. Föster resonace energy transfer—A spectroscopic nanoruler: Principle and application. J. Photochem. Photobiol. C: Photochem. Rev 2011, 12, 20–30. [Google Scholar]
- Clegg, R.M. Förster resonance energy transfer—FRET what is it, why do it, and how it’s done. Lab. Tech. Biochem. Mol. Biol 2009, 33, 1–57. [Google Scholar]
- Liu, C.P.; Cheng, S.H.; Chen, N.T.; Lo, L.W. Intra/inter-particle energy transfer of luminescence nanocrystals for biomedical applications. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Kim, G.B.; Kim, Y.P. Analysis of protease activity using quantum dots and resonance energy transfer. Theranostics 2012, 2, 127–138. [Google Scholar]
- Jares-Erijman, E.A.; Jovin, T.M. Imaging molecular interactions in living cells by FRET microscopy. Curr. Opin. Chem. Biol 2006, 10, 409–416. [Google Scholar]
- Jares-Erijman, E.A.; Jovin, T.M. FRET imaging. Nat. Biotechnol 2003, 21, 1387–1395. [Google Scholar]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for fluorescence resonance energy transfer analysis: Beyond traditional donor-acceptor combinations. Angew. Chem. Int. Ed 2006, 45, 4562–4588. [Google Scholar]
- Verma, P.K.; Pala, S.K. Ultrafast resonance energy transfer in bio-molecular systems. Eur. Phys. J. D 2010, 60, 137–156. [Google Scholar]
- Hildebrandt, N.; Charbonniere, L.J.; Beck, M.; Ziessel, R.F.; Lohmannsroben, H.G. Quantum dots as efficient energy acceptors in a time-resolved fluoroimmunoassay. Angew. Chem 2005, 117, 7784–7788. [Google Scholar]
- Schuler, B.; Eaton, W.A. Protein folding studied by single-molecule FRET. Curr. Opin. Struct. Biol 2008, 18, 16–26. [Google Scholar] [Green Version]
- Hillisch, A.; Lorenz, M.; Diekmann, S. Recent advances in FRET: Distance determination in protein—DNA complexes. Curr. Opin. Struct. Biol 2001, 11, 201–207. [Google Scholar]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev 2009, 38, 1759–1782. [Google Scholar]
- Dubertret, B.; Calame, M.; Libchaber, A.J. Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat. Biotechnol 2001, 19, 365–370. [Google Scholar]
- Zhang, Y.; Wang, T.-H. Quantum dot enabled molecular sensing and diagnostics. Theranostics 2012, 2, 631–654. [Google Scholar]
- Bruchez, M., Jr; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar]
- Reiss, P.; Protiere, M.; Li, L. Core/shell semiconductor nanocrystals. Small 2009, 5, 154–168. [Google Scholar]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar]
- Ho, Y.P.; Chen, H.H.; Leong, K.W.; Wang, T.H. Evaluating the intracellular stability and unpacking of DNA nanocomplexes by quantum dots-FRET. J. Control. Release 2006, 116, 83–89. [Google Scholar]
- Lee, J.; Choi, Y.; Kim, J.; Park, E.; Song, R. Positively charged compact quantum dot-DNA complexes for detection of nucleic acids. Chem. Phys. Chem 2009, 10, 806–811. [Google Scholar]
- Chi, C.W.; Lao, Y.H.; Li, Y.S.; Chen, L.C. A quantum dot-aptamer beacon using a DNA intercalating dye as the FRET reporter: Application to label-free thrombin detection. Biosens. Bioelectron 2011, 26, 3346–3352. [Google Scholar]
- Huang, Y.; Zhao, S.L.; Shi, M.; Chen, J.; Chen, Z.F.; Liang, H. Intermolecular and intramolecular quencher based quantum dot nanoprobes for multiplexed detection of endonuclease activity and inhibition. Anal. Chem 2011, 83, 8913–8918. [Google Scholar]
- Medintz, I.L.; Clapp, A.R.; Mattoussi, H.; Goldman, E.R.; Fisher, B.; Mauro, J.M. Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat. Mater 2003, 2, 630–638. [Google Scholar]
- Geissbuehler, I.; Hovius, R.; Martinez, K.L.; Adrian, M.; Thampi, K.R.; Vogel, H. Lipid-coated nanocrystals as multifunctionalized luminescent scaffolds for supramolecular biological assemblies. Angew. Chem. Int. Ed 2005, 44, 1388–1392. [Google Scholar]
- Lu, H.; Schöps, O.; Woggon, U.; Niemeyer, C.M. Self-assembled donor comprising quantum dots and fluorescent proteins for long-range fluorescence resonance energy transfer. J. Am. Chem. Soc 2008, 130, 4815–4827. [Google Scholar]
- Chen, H.H.; Ho, Y.P.; Jiang, X.; Mao, H.Q.; Wang, T.H.; Leong, K.W. Quantitative comparison of intracellular unpacking kinetics of polyplexes by a model constructed from quantum dot-FRET. Mol. Ther 2008, 16, 324–332. [Google Scholar]
- Chen, H.H.; Ho, Y.P.; Jiang, X.; Mao, H.Q.; Wang, T.H.; Leong, K.W. Simultaneous non invasive analysis of DNA condensation and stability by two-step QD-FRET. Nano Today 2009, 4, 125–134. [Google Scholar]
- Jiang, G.; Susha, A.S.; Lutich, A.A.; Stefani, F.D.; Feldmann, J.; Rogach, A.L. Cascaded FRET in conjugated polymer/quantum dot/dye-labeled DNA complexes for DNA hybridization detection. ACS Nano 2009, 3, 4127–4131. [Google Scholar]
- Boeneman, K.; Prasuhn, D.E.; Blanco-Canosa, J.B.; Dawson, P.E.; Melinger, J.S.; Ancona, M.; Stewart, M.H.; Susumu, K.; Huston, A.; Medintz, I.L. Self-assembled quantum dot-sensitized multivalent DNA photonic wires. J. Am. Chem. Soc 2010, 132, 18177–18190. [Google Scholar]
- Algar, W.R.; Wegner, D.; Huston, A.L.; Blanco-Canosa, J.B.; Stewant, M.H.; Armstrong, A.; Dawson, P.E.; Hildebrandt, N.; Medintz, I.L. Quantum dots as simultaneous acceptors and donors in time-gated Förster resonance energy transfer relays: Characterization and biosensing. J. Am. Chem. Soc 2012, 134, 1876–1891. [Google Scholar]
- Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev 2004, 104, 139–173. [Google Scholar]
- Kuningas, K.; Rantanen, T.; Ukonaho, T.; Lovgren, T.; Soukka, T. Homogeneous assay technology based on upconverting phosphors. Anal. Chem 2005, 77, 7348–7355. [Google Scholar]
- Kuningas, K.; Ukonaho, T.; Päkkilä, H.; Rantanen, T.; Rosenberg, J.; Lövgren, T.; Soukka, T. Upconversion fluorescence resonance energy transfer in a homogeneous immunoassay for estradiol. Anal. Chem 2006, 78, 4690–4696. [Google Scholar]
- Bednarkiewicz, A.; Nyk, M.; Samoc, M.; Strek, W. Up-conversion FRET from Er3+/Yb3+:NaYF4 nanophosphor to CdSe quantum dots. J. Phys. Chem. C 2010, 114, 17535–17541. [Google Scholar]
- Rantanen, T.; Järvenpää, M.L.; Vuojola, J.; Kuningas, K.; Soukka, T. Fluorescence-quenching-based enzyme-activity assay by using photon upconversion. Angew. Chem. Int. Ed 2008, 47, 3811–3813. [Google Scholar]
- Vetrone, F.; Naccache, R.; Morgan, C.G.; Capobianco, J.A. Luminescence resonance energy transfer from an upconverting nanoparticle to a fluorescent phycobiliprotein. Nanoscale 2010, 2, 1185–1189. [Google Scholar]
- Guo, H.C.; Idris, N.M.; Zhang, Y. LRET-based biodetection of DNA release in live cells using surface-modified upconverting fluorescent nanoparticles. Langmuir 2011, 27, 2854–2860. [Google Scholar]
- Wang, L.; Yan, R.; Huo, Z.; Wang, L.; Zeng, J.; Bao, J.; Wang, X.; Peng, Q.; Li, Y. Fluorescence resonant energy transfer biosensor based on upconversion-luminescent nanoparticles. Angew. Chem. Int. Ed 2005, 44, 6054–6057. [Google Scholar]
- Wang, M.; Hou, W.; Mi, C.C.; Wang, W.X.; Xu, Z.R.; Teng, H.H.; Mao, C.B.; Xu, S.K. Immunoassay of goat antihuman immunoglobulin G antibody based on luminescence resonance energy transfer between near-infrared responsive NaYF4:Yb, Er Upconversion fluorescent nanoparticles and gold nanoparticles. Anal. Chem 2009, 81, 8783–8789. [Google Scholar]
- Lu, C.H.; Yang, H.H.; Zhu, C.L.; Chen, X.; Chen, G.N. A graphene platform for sensing biomolecules. Angew. Chem., Int. Ed 2009, 48, 4785–4787. [Google Scholar]
- Dong, H.; Gao, W.; Yan, F.; Ji, H.; Ju, H. Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal. Chem 2010, 82, 5511–5517. [Google Scholar]
- Wu, W.; Hu, H.; Li, F.; Wang, L.; Gao, J.; Lu, J.; Fan, C. A graphene oxide-based nano-beacon for DNA phosphorylation analysis. Chem. Commun 2011, 47, 1201–1203. [Google Scholar]
- Chang, H.; Tang, L.; Wang, Y.; Jiang, J.; Li, J. Graphene fluorescence resonance energy transfer aptasensor for the thrombin detection. Anal. Chem 2010, 82, 2341–2346. [Google Scholar]
- Jang, H.; Kim, Y.K.; Kwon, H.M.; Yeo, W.S.; Kim, D.E.; Min, D.H. A graphene-based platform for the assay of duplex-DNA unwinding by helicase. Angew. Chem. Int. Ed 2010, 49, 5703–5707. [Google Scholar]
- Liu, M.; Zhao, H.; Quan, X.; Chen, S.; Fan, X. Distance-independent quenching of quantum dots by nanoscale-graphene in self-assembled sandwich immunoassay. Chem. Commun 2010, 46, 7909–7911. [Google Scholar]
- Zhang, C.; Yuan, Y.; Zhang, S.; Wang, Y.; Liu, Z. Biosensing platform based on fluorescence resonance energy transfer from upconverting nanocrystals to graphene Oxide. Angew. Chem. Int. Ed 2011, 50, 6851–6854. [Google Scholar]
- Liu, C.H.; Wang, Z.; Jia, H.X.; Li, Z.P. Efficient fluorescence resonance energy transfer between upconversion nanophosphors and graphene oxide: A highly sensitive biosensing platform. Chem. Commun 2011, 47, 4661–4663. [Google Scholar]
- Wu, S.; Duan, N.; Ma, X.Y.; Xia, Y.; Wang, H.X.; Wang, Z.P.; Zhang, Q. Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal. Chem 2012, 84, 6263–6270. [Google Scholar]
- Wang, Y.H.; Bao, L.; Liu, Z.H.; Pang, D.W. Aptamer biosensor based on fluorescence resonance energy transfer from upconverting phosphors to carbon nanoparticles for thrombin detection in human plasma. Anal. Chem 2011, 83, 8130–8137. [Google Scholar]
- Xu, H.; Hepel, M. “Molecular beacon”-based fluorescent assay for selective detection of glutathione and cysteine. Anal. Chem 2011, 83, 813–819. [Google Scholar]
- Huang, Y.; Zhao, S.L.; Liang, H.; Chen, Z.F.; Liu, Y.M. Multiplex detection of endonucleases by using a multicolor gold nanobeacon. Chem-A Eur. J 2011, 17, 7313–7319. [Google Scholar]
- Ray, P.C.; Darbha, G.K.; Ray, A.; Walker, J.; Hardy, W. Gold nanoparticle based FRET for DNA detection. Plasmonics 2007, 2, 173–183. [Google Scholar]
- Lee, S.; Cha, E.-J.; Park, K.; Lee, S.-Y.; Hong, J.-K.; Sun, I.-C.; Kim, S.Y.; Choi, K.; Kwon, I.C.; Kim, K.; et al. A near-infrared-fluorescencequenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew. Chem. Int. Ed 2008, 47, 2804–2807. [Google Scholar]
- Cheng, Y.N.; Stakenborg, T.; Van Dorpe, P.; Lahae, L.; Wang, M.; Chen, H.Z.; Borghs, G. Fluorescence near gold nanoparticles for DNA sensing. Anal. Chem 2011, 83, 1307–1314. [Google Scholar]
- Feng, J.-J.; Zhoa, G.; Xu, J.-J.; Chen, H.-Y. Direct electrochemistry and electrocatalysis of heme proteins immobilized on gold nanoparticles stabilized by chitosan. Anal. Biochem 2005, 342, 280–286. [Google Scholar]
- Yang, T.; Li, Z.; Wang, L.; Guo, C.; Sun, Y. Synthesis, characterization, and self-assembly of protein lysozyme monolayer-stabilized gold nanoparticles. Langmuir 2007, 23, 10533–10538. [Google Scholar]
- Mattoussi, H.; Mauro, J.M.; Goldman, E.R.; Anderson, G.P.; Sundar, V.C.; Mikulec, F.B.; Bawendi, M.G. Self-Assembly of CdSe–ZnS quantum dot bioconjugates using an engineered recombinant protein. J. Am. Chem. Soc 2000, 122, 12142–12150. [Google Scholar]
- Goldman, E.R.; Anderson, G.P.; Tran, P.T.; Mattoussi, H.; Charles, P.T.; Mauro, J.M. Conjugation of luminescent quantum dots with antibodies using an engineered adaptor protein to provide new reagents for fluoroimmunoassays. Anal. Chem 2002, 74, 841–847. [Google Scholar]
- Jaiswal, J.K.; Mattoussi, H.; Mauro, J.M.; Simon, S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol 2003, 21, 47–51. [Google Scholar]
- Mandal, G.; Bardhan, M.; Ganguly, T. Occurrence of Förster resonance energy transfer between quantum dots and gold nanoparticles in the presence of a biomolecule. J. Phys. Chem. C 2011, 115, 20840–20848. [Google Scholar]
- He, X.; Wang, K.; Cheng, Z. In vivo near-infrared fluorescence imaging of cancer with nanoparticle-based probes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2010, 2, 349–366. [Google Scholar]
- Maysinger, D.; Lovric, J.; Eisenberg, A.; Savic, R. Fate of micelles and quantum dots in cells. Eur. J. Pharm. Biopharm 2007, 65, 270–281. [Google Scholar]
- Huang, X.; Lee, S.; Chen, X. Design of “smart” probes for optical imaging of apoptosis American journal of nuclear medicine and molecular imaging. Am. Nuclear Med. Mol. Imaging 2011, 1, 3–17. [Google Scholar]
- Hering, V.R.; Gibson, G.; Schumacher, R.I.; Faljoni-Alario, A.; Politi, M.J. Energy transfer between CdSe/ZnS core/shell quantum dots and fluorescent proteins. Bioconjugate Chem 2007, 18, 1705–1708. [Google Scholar]
- Shaheen, S.M.; Akita, H.; Yamashita, A.; Katoono, R.; Yui, N.; Biju, V.; Ishikawa, M.; Harashima, H. Quantitative analysis of condensation/decondensation status of pDNA in the nuclear sub-domains by QD-FRET. Nucleic Acids Res 2011, 39, e48. [Google Scholar]
- Ghadiali, J.E.; Cohen, B.E.; Stevens, M.M. Protein kinase-actuated resonance energy transfer in quantum dot-peptide conjugates. ACS Nano 2010, 4, 4915–4919. [Google Scholar]
- Dennis, A.M.; Rhee, W.J.; Sotto, D.; Dublin, S.N.; Bao, G. Quantum dot-fluorescent protein FRET probes for sensing intracellular pH. ACS Nano 2012, 6, 2917–2924. [Google Scholar]
- Xue, M.; Wang, X.; Wang, H.; Chen, D.; Tang, B. Hydrogen bond breakage by fluoride anions in a simple CdTe quantum dot/gold nanoparticle FRET system and its analytical application. Chem. Commun 2011, 47, 4986–4988. [Google Scholar]
- Xue, M.; Wang, X.; Duan, L.; Gao, W.; Ji, L.; Tang, B. A new nanoprobe based on FRET between functional quantum dots and gold nanoparticles for fluoride anion and its applications for biological imaging. Biosens. Bioelectron 2012, 36, 168–173. [Google Scholar]
- Skajaa, T.; Zhao, Y.; van den Heuvel, D.J.; Gerritsen, H.C.; Cormode, D.P.; Koole, R.; van Schooneveld, M.M.; Post, J.A.; Fisher, E.A.; Fayad, Z.A. Quantum dot and cy5.5 labeled nanoparticles to investigate lipoprotein biointeractions via Förster resonance energy transfer. Nano Lett 2010, 10, 5131–5138. [Google Scholar]
- Boeneman, K.; Deschamps, J.R.; Buckhout-White, S.; Prasuhn, D.E.; Blanco-Canosa, J.B.; Dawson, P.E.; Stewart, M.H.; Susumu, K.; Goldman, E.R.; Ancona, M. Quantum dot DNA bioconjugates: Attachment chemistry strongly influences the resulting composite architecture. ACS Nano 2010, 4, 7253–7266. [Google Scholar]
- Modi, S.; M, G.S.; Goswami, D.; Gupta, G.D.; Mayor, S.; Krishnan, Y. A DNA nanomachine that maps spatial and temporal pH changes inside living cells. Nat. Nanotechnol 2009, 4, 325–330. [Google Scholar]
- Surana, S.; Bhat, J.M.; Koushika, S.P.; Krishnan, Y. An autonomous DNA nanomachine maps spatiotemporal pH changes in a multicellular living organism. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef]
- Wagh, A.; Qian, S.Y.; Law, B. Development of Biocompatible Polymeric Nanoparticles for in Vivo NIR and FRET Imaging. Bioconjugate Chem 2012, 23, 981–992. [Google Scholar]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. [Google Scholar]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion nanoparticles for potential cancer theranostics. Ther. Deliv 2011, 2, 1235–1239. [Google Scholar]
- Cheng, L.; Yang, K.; Li, Y.; Chen, J.; Wang, C.; Shao, M.; Lee, S.T.; Liu, Z. Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy. Angew. Chem. Int. Ed. Engl 2011, 50, 7385–7390. [Google Scholar]
- Cheng, L.; Yang, K.; Li, Y.; Zeng, X.; Shao, M.; Lee, S.T.; Liu, Z. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials 2012, 33, 2215–2222. [Google Scholar]
- Xiong, L.Q.; Chen, Z.G.; Tian, Q.W.; Cao, T.Y.; Xu, C.J.; Huang, C.H. High Contrast upconversion luminescence targeted imaging in vivo using peptide-labeled nanophosphors. Anal. Chem 2009, 81, 8687–8694. [Google Scholar]
- Liu, Q.; Peng, J.; Sun, L.; Li, F. High-efficiency upconversion luminescent sensing and bioimaging of Hg(II) by chromophoric ruthenium complex-assembled nanophosphors. ACS Nano 2011, 5, 8040–8048. [Google Scholar]
- Cheng, L.; Yang, K.; Shao, M.; Lee, S.T.; Liu, Z. Multicolor in vivo imaging of upconversion nanoparticles with emissions tuned by luminescence resonance energy transfer. J. Phys. Chem. C 2011, 115, 2686–2692. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol 2007, 2, 751–760. [Google Scholar]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J 2005, 19, 311–330. [Google Scholar]
- Bonoiu, A.; Mahajan, S.D.; Ye, L.; Kumar, R.; Ding, H.; Yong, K.-T. MMP-9 gene silencing by a quantum dot-siRNA nanoplex delivery to maintain the integrity of the blood brain barrier. Brain Res 2009, 1282, 142–155. [Google Scholar]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett 2010, 10, 3223–3230. [Google Scholar]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett 2008, 8, 2906–2912. [Google Scholar]
- Shen, X.Q.; Li, L.; Wu, H.; Yao, S.Q.; Xu, Q.H. Photosensitizer-doped conjugated polymer nanoparticles for simultaneous two-photon imaging and two-photon photodynamic therapy in living cells. Nanoscale 2011, 3, 5140–5146. [Google Scholar]
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv. Drug Deli. Rev 2001, 46, 125–148. [Google Scholar]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng 2010, 1, 149–173. [Google Scholar]
- Alexis, F.; Rhee, J.W.; Richie, J.P.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. New frontiers in nanotechnology for cancer treatment. Urol. Oncol 2008, 26, 74–85. [Google Scholar]
- Bharali, D.J.; Klejbor, I.; Stachowiak, E.K.; Dutta, P.; Roy, I.; Kaur, N. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl. Acad. Sci. USA 2005, 102, 11539–11544. [Google Scholar]
- Ding, H.; Yong, K.T.; Roy, I.; Pudavar, H.E.; Law, W.C.; Bergey, E.J. Gold nanorods coated with multilayer polyelectrolyte as contrast agents for multimodal imaging. J. Phys. Chem. C 2007, 111, 12552–12557. [Google Scholar]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett 2005, 5, 829–834. [Google Scholar]
- Ballou, B.; Ernst, L.A.; Andreko, S.; Harper, T.; Fitzpatrick, J.A.J.; Waggoner, A.S.; Bruchez, M.P. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconjugate Chem 2007, 18, 389–396. [Google Scholar]
- Carling, C.J.; Nourmohammadian, F.; Boyer, J.C.; Branda, N.R. Remote-control photorelease of caged compounds using near-infrared light and upconverting nanoparticles. Angew. Chem. Int. Ed 2010, 49, 3782–3785. [Google Scholar]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci 2007, 32, 962–990. [Google Scholar]
- Ulbrich, K.; Etrych, T.; Chytil, P.; Jelinkova, M.; Rihova, B. Antibody-targeted polymer-doxorubicin conjugates with pH-controlled activation. J. Drug Target 2004, 12, 477–489. [Google Scholar]
- Schafer, F.Q.; Buettner, G.R. Redox state as viewed through the glutathione disulfide/glutathione couple. Free Radic. Biol. Med 2001, 30, 1191–1212. [Google Scholar]
- Park, C.; Kim, H.; Kim, S.; Kim, C. Enzyme responsive nanocontainers with cyclodextrin gatekeepers and synergistic effects in release of guests. J. Am. Chem. Soc 2009, 131, 16614–16615. [Google Scholar]
- Medintz, I.L.; Mattoussi, H. Quantum dot-based resonance energy transfer and its growing application in biology. Phys. Chem. Chem. Phys 2009, 11, 17–45. [Google Scholar]
- Algar, W.R.; Krull, U.J. Quantum dots as donors in fluorescence resonance energy transfer for the bioanalysis of nucleic acids, proteins, and other biological molecules. Anal. Bioanal. Chem 2008, 391, 1609–1618. [Google Scholar]
- Xu, H.X.; Sha, M.Y.; Wong, E.Y.; Uphoff, J.; Xu, Y.H.; Treadway, J.A.; Truong, A.; O’Brien, E.; Asquith, S.; Stubbins, M.; et al. Multiplexed SNP genotyping using the Qbead™ system: A quantum dotencoded microspherebased assay. Nucleic Acids Res 2003, 31, e43. [Google Scholar]
- Ho, Y.P.; Kung, M.C.; Yang, S.; Wang, T.H. Multiplexed hybridization detection with multicolor colocalization of quantum dot nanoprobes. Nano Lett 2005, 5, 1693–1697. [Google Scholar]
- Choi, H.S; Frangioni, J.V. Nanoparticles for biomedical imaging: Fundamentals of clinical translation. Mol. Imaging. 2010, 9, 291–310. [Google Scholar]
- Rosenholm, J.M.; Sahlgren, C.; Linden, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles—opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar]
- Shubayev, V.I.; Pisanic, T.R., 2nd; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev 2009, 61, 467–477. [Google Scholar]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res 2010, 62, 90–99. [Google Scholar]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labeling and sensing. Nat. Mater 2005, 4, 435–446. [Google Scholar]
- Zhang, C.Y.; Yeh, H.C.; Kuroki, M.T.; Wang, T.H. Single-quantum-dot-based DNA nanosensor. Nat. Mater 2005, 4, 826–831. [Google Scholar]
- Bagalkot, V.; Zhang, L.; Levy-Nissenbaum, E.; Jon, S.; Kantoff, P.W; Langer, R.; Farokhzad, O.C. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett 2007, 7, 3065–3070. [Google Scholar]
- Xu, G.; Yong, K.T.; Roy, I.; Mahajan, S.D.; Ding, H.; Schwartz, S.A. Bioconjugated quantum rods as targeted probes for efficient transmigration across an in vitro blood-brain barrier. Bioconjugate Chem 2008, 19, 1179–1185. [Google Scholar]
- Chen, A.A.; Derfus, A.M.; Khetani, S.R.; Bhatia, S.N. Quantum dots to monitor RNAi delivery and improve gene silencing. Nucleic Acids Res 2005, 33, e190. [Google Scholar]
- Tan, W.B.; Jiang, S.; Zhang, Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials 2007, 28, 1565–1571. [Google Scholar]
- Qi, L.; Gao, X. Quantum dot-amphipol nanocomplex for intracellular delivery and real-time imaging of siRNA. ACS Nano 2008, 2, 1403–1410. [Google Scholar]
- Lee, H.; Kim, I.-K.; Park, T.G. Synthesis, characterization, and in vivo diagnostic applications of hyaluronic acid immobilized gold nanoprobes. Bioconjugate Chem 2010, 21, 289–295. [Google Scholar]
- Gopalakrishnan, G.; Danelon, C.; Izewska, P.; Prummer, M.; Bolinger, P.Y.; Geissbuhler, I.; Demurtas, D.; Dubochet, J.; Vogel, H. Multifunctional lipid/quantum dot hybrid nanocontainers for controlled targeting of live cells. Angew. Chem., Int. Ed 2006, 45, 5478–483. [Google Scholar]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Bomans, P.H.; Frederik, P.M.; Kostarelos, K. Functionalized-quantum-dot-liposome hybrids as multimodal nanoparticles for cancer. Small 2008, 4, 1406–1415. [Google Scholar]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Tian, B.; Cakebread, A.; Halket, J.M.; Kostarelos, K. Tumor targeting of functionalized quantum dot-liposome hybrids by intravenous administration. Mol. Pharm 2009, 6, 520–530. [Google Scholar]
- Weng, K.C.; Noble, C.O.; Papahadjopoulos-Sternberg, B.; Chen, F.F.; Drummond, D.C.; Kirpotin, D.B.; Wang, D.; Hom, Y.K.; Hann, B.; Park, J.W. Targeted tumor cell internalization and imaging of multifunctional quantum dot-conjugated immunoliposomes in vitro and in vivo. Nano Lett. 2008, 8, 2851–2857. [Google Scholar]
- Tian, B.; Al-Jamal, W.T.; Al-Jamal, K.T.; Kostarelos, K. Doxorubicin-loaded lipid-quantum dot hybrids: Surface topography and release properties. Int. J. Pharm 2011, 416, 443–447. [Google Scholar]
- Wu, W.; Aiello, M.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. In-situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials 2010, 31, 3023–3031. [Google Scholar]
- Chen, H.; Kim, S.; Li, L.; Wang, S.; Park, K.; Cheng, J.X. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Förster resonance energy transfer imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 6596–6601. [Google Scholar]
- Chen, K.J.; Chiu, Y.L.; Chen, Y.M.; Ho, Y.C.; Sung, H.W. Intracellularly monitoring/imaging the release of doxorubicin from pH-responsive nanoparticles using Förster resonance energy transfer. Biomaterials 2011, 32, 2586–2592. [Google Scholar]
- Banerjee, S.S.; Chen, D.H. Multifunctional pH-sensitive magnetic nanoparticles for simultaneous imaging, sensing and targeted intracellular anticancer drug delivery. Nanotechnology 2008, 19, 555104. [Google Scholar]
- Lee, C.H.; Cheng, S.H.; Huang, I.P.; Souris, J.S.; Yang, C.S.; Mou, C.Y.; Lo, L.W. Intracellular pH-responsive mesoporous silica nanoparticles for controlled release of anticancer chemotherapeutics. Angew. Chem. Int. Ed. Engl 2010, 49, 8214–8219. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, N.-T.; Cheng, S.-H.; Liu, C.-P.; Souris, J.S.; Chen, C.-T.; Mou, C.-Y.; Lo, L.-W. Recent Advances in Nanoparticle-Based Förster Resonance Energy Transfer for Biosensing, Molecular Imaging and Drug Release Profiling. Int. J. Mol. Sci. 2012, 13, 16598-16623. https://doi.org/10.3390/ijms131216598
Chen N-T, Cheng S-H, Liu C-P, Souris JS, Chen C-T, Mou C-Y, Lo L-W. Recent Advances in Nanoparticle-Based Förster Resonance Energy Transfer for Biosensing, Molecular Imaging and Drug Release Profiling. International Journal of Molecular Sciences. 2012; 13(12):16598-16623. https://doi.org/10.3390/ijms131216598
Chicago/Turabian StyleChen, Nai-Tzu, Shih-Hsun Cheng, Ching-Ping Liu, Jeffrey S. Souris, Chen-Tu Chen, Chung-Yuan Mou, and Leu-Wei Lo. 2012. "Recent Advances in Nanoparticle-Based Förster Resonance Energy Transfer for Biosensing, Molecular Imaging and Drug Release Profiling" International Journal of Molecular Sciences 13, no. 12: 16598-16623. https://doi.org/10.3390/ijms131216598



