Isolation and Characterization of Squamous Cell Carcinoma-Derived Stem-like Cells: Role in Tumor Formation
Abstract
:1. Introduction
2. Results and Discussion
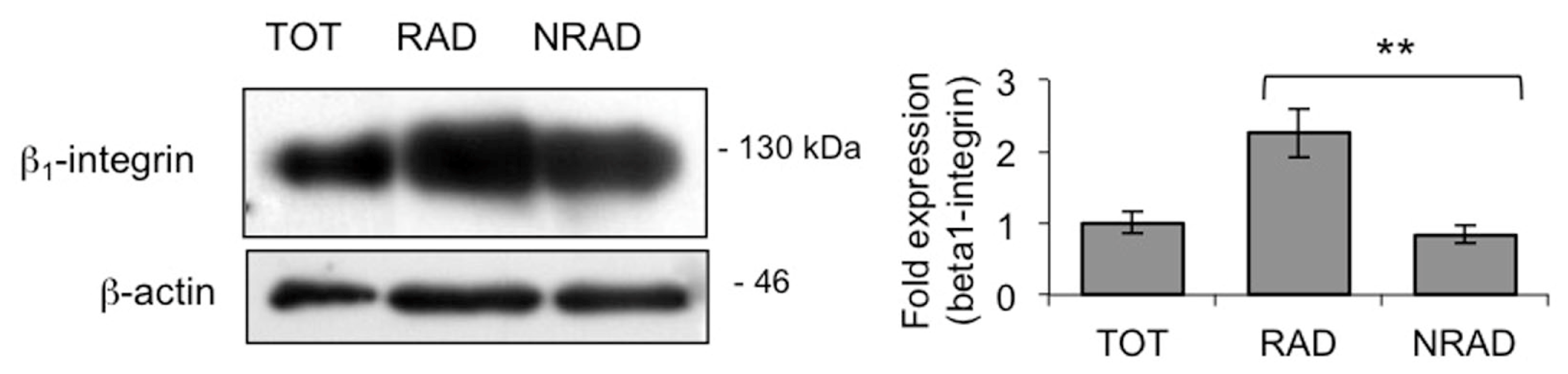
2.1. RAD Cells from Primary cSCC Cultures Are Abundant and Express High Levels of β1-Integrin
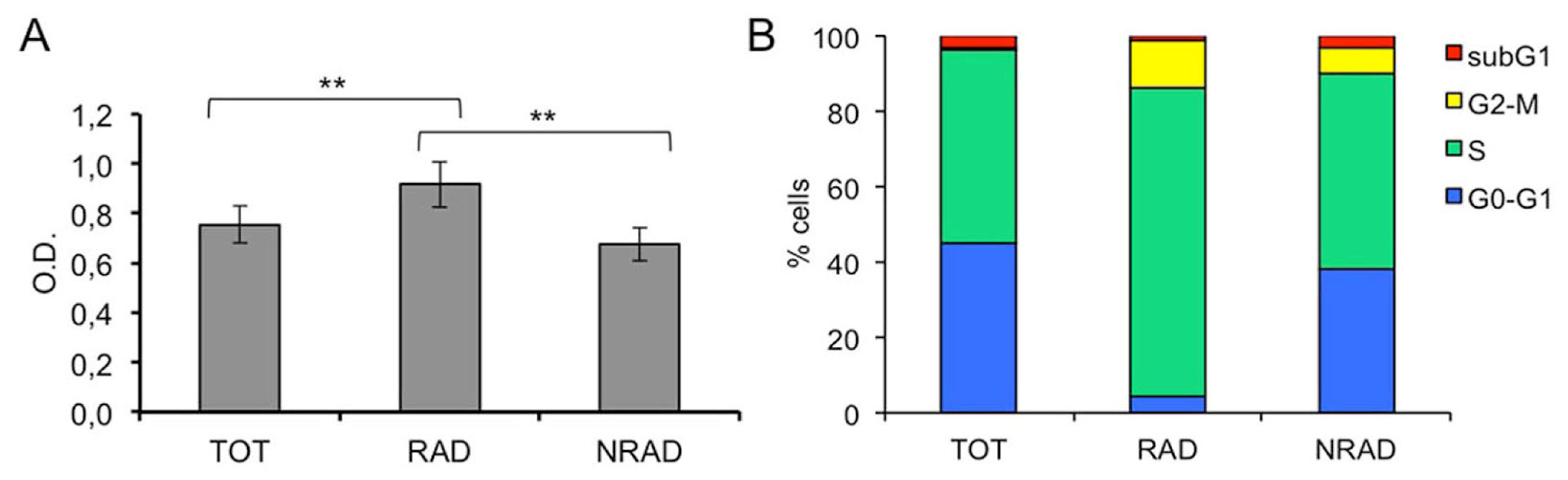
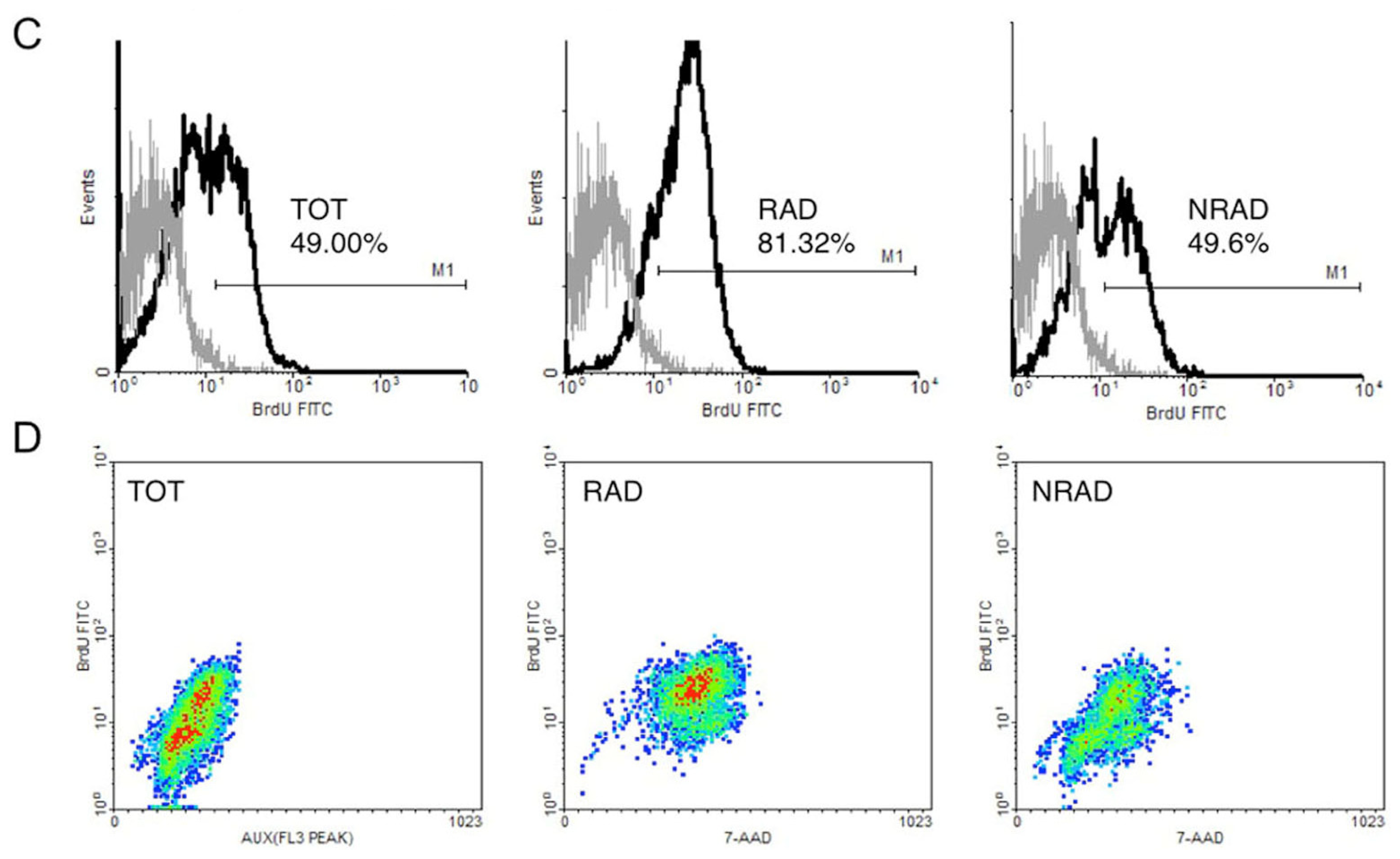
2.2. RAD from cSCC Are Highly Proliferating Cells In Vitro
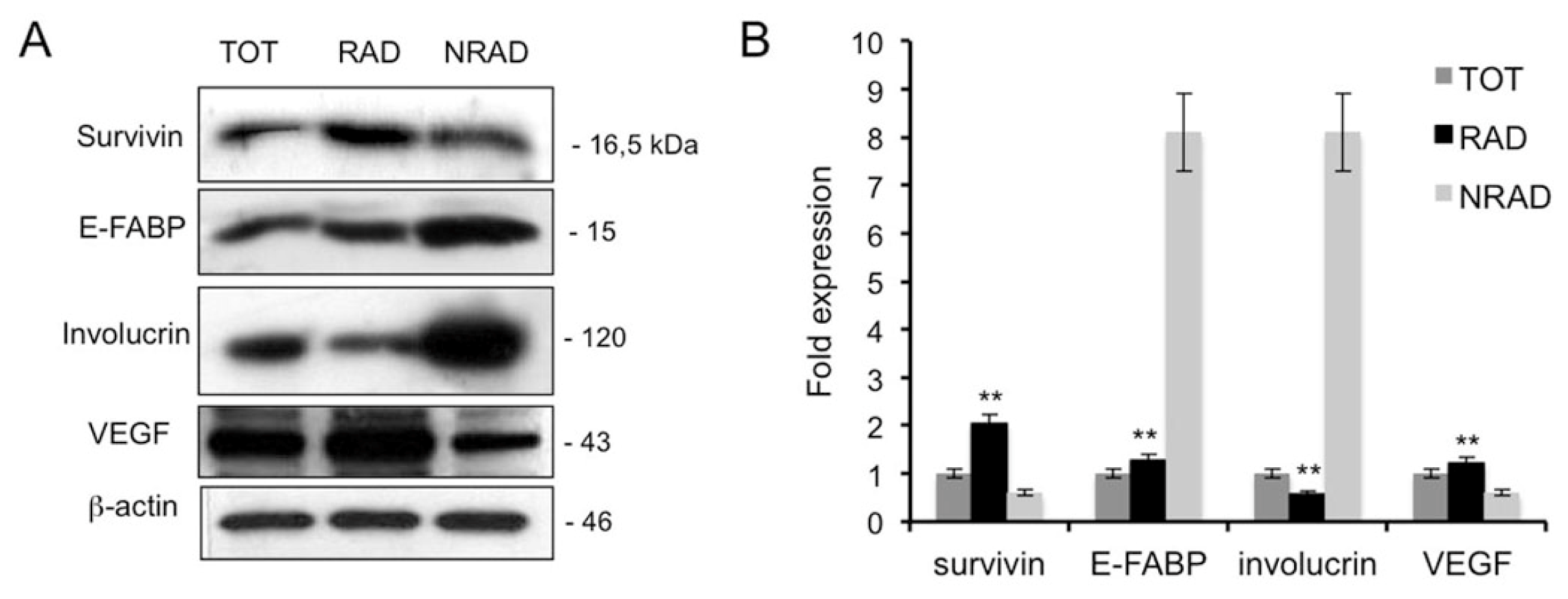
2.3. RAD cSCC Cells Are Less Differentiated and Express High Levels of Survivin
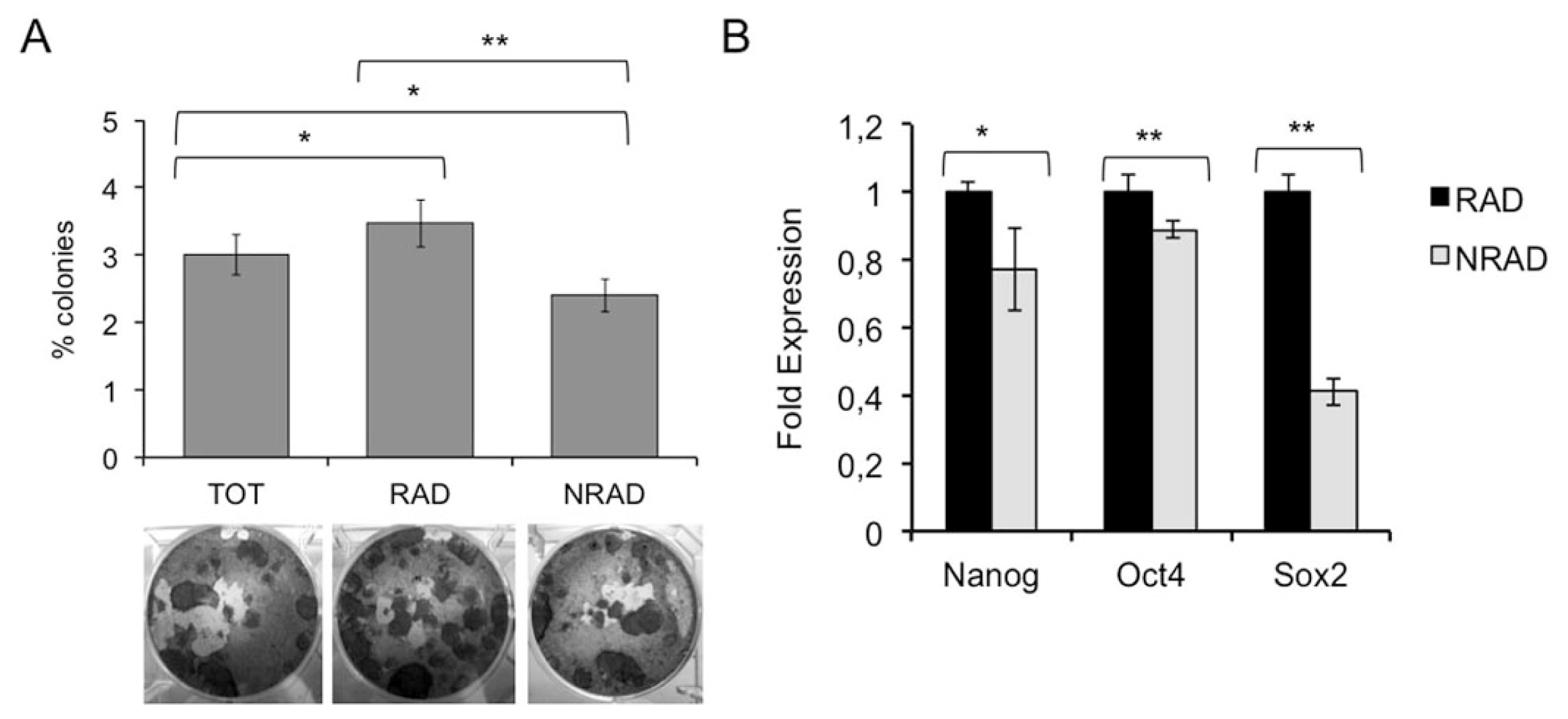
2.4. RAD Cells from cSCC Display High Colony Forming Efficiency and Increased Expression of Stem Cell-Associated Genes
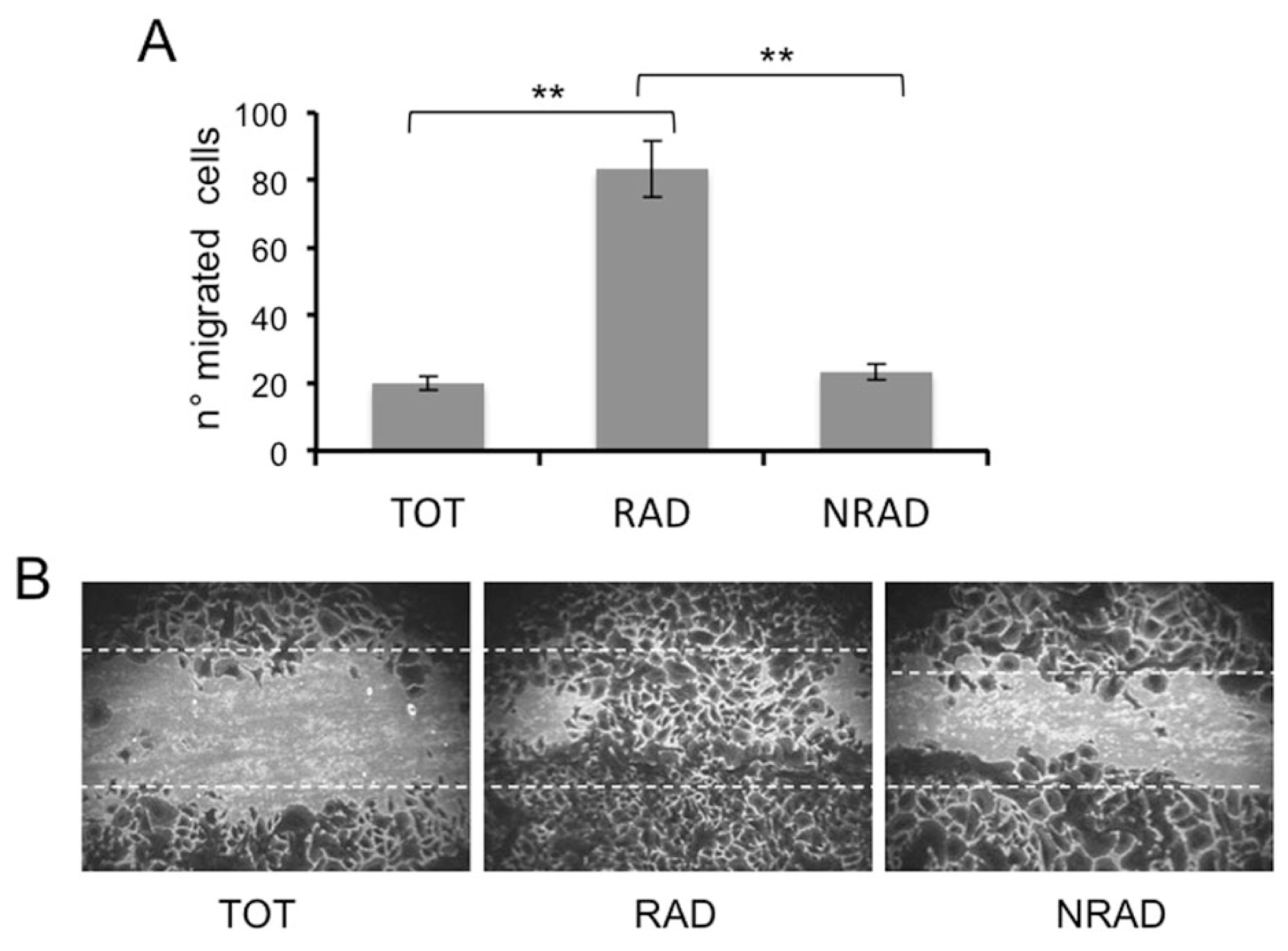
2.5. RAD Cells Migrate to a Greater Extent than NRAD and Total SCC Keratinocytes
2.6. RAD Cells from cSCCs Are Tumorigenic In Vivo
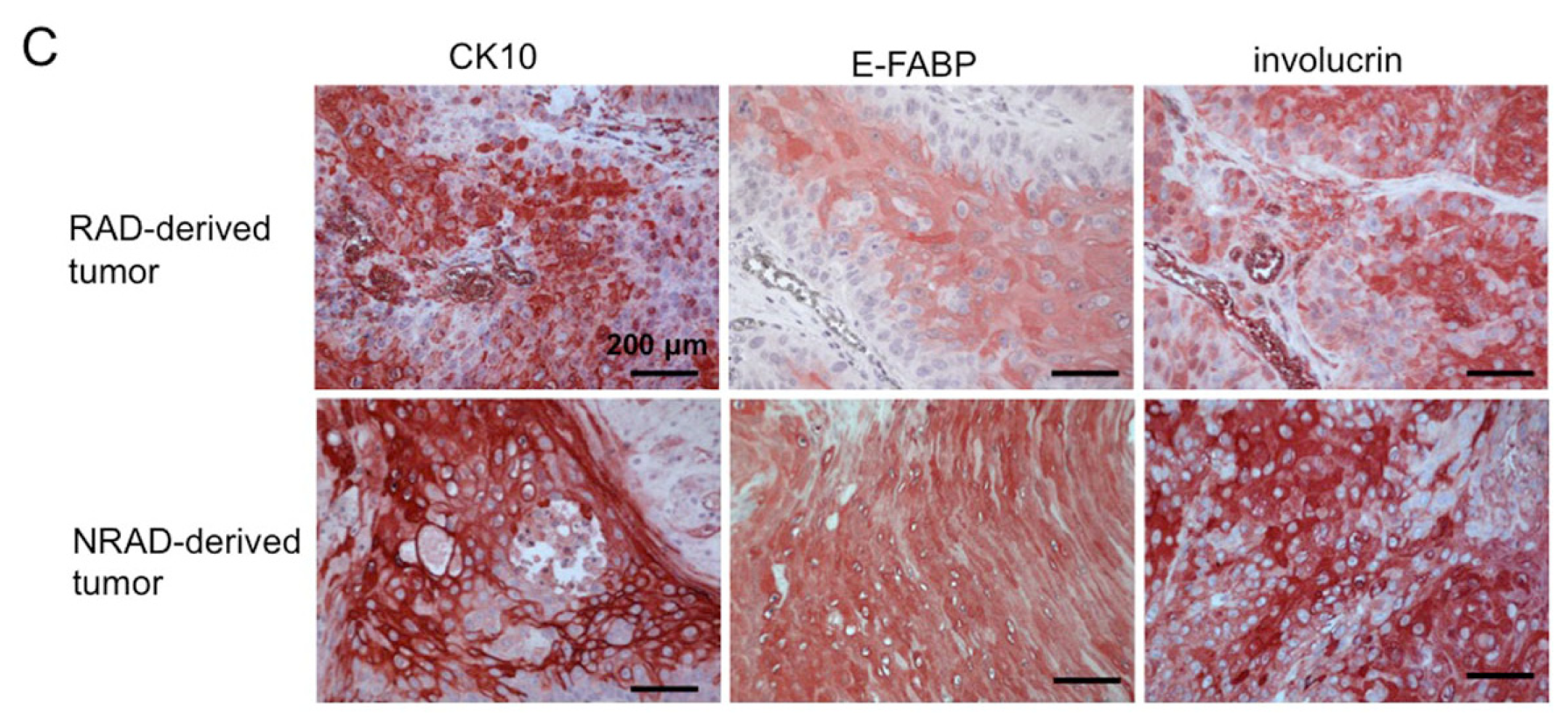
2.7. RAD-Derived Tumors Display Most Aggressive Features
3. Experimental Section
3.1. Isolation of Primary Keratinocytes from cSCCs Tissues
3.2. Western Blotting
3.3. Detection of Cell Viability by Crystal Violet Staining
3.4. BrdU Proliferation Assay
3.5. Colony Forming Efficiency (CFE)
3.6. Scratching Assay
3.7. H&E Staining and Mitotic Index Calculation
3.8. In Vivo Tumorigenesis
3.9. Immunohistochemistry
3.10. Real Time PCR
4. Conclusions


Acknowledgments
Conflicts of Interest
References
- Lohmann, C.M.; Solomon, A.R. Clinicopathologic variants of cutaneous squamous cell carcinoma. Adv. Anat. Pathol 2001, 8, 27–36. [Google Scholar]
- Morris, R.J. Keratinocyte stem cells: targets for cutaneous carcinogens. J. Clin. Invest 2000, 106, 3–8. [Google Scholar]
- Lapouge, G.; Youssef, K.K.; Vokaer, B.; Achouri, Y.; Michaux, C.; Sotiropoulou, P.A.; Blanpain, C. Identifying the cellular origin of squamous skin tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 7431–7436. [Google Scholar]
- Jones, P.H.; Watt, F.M. Separation of human epidermal stem cells from transit amplyfying cells on the basis of differences in integrin function and expression. Cell 1993, 73, 713–724. [Google Scholar]
- Tan, D.W.; Jensen, K.B.; Trotter, M.W.; Connelly, J.T.; Broad, S.; Watt, F.M. Single-cell gene expression profiling reveals functional heterogeneity of undifferentiated human epidermal cells. Development 2013, 140, 1433–1444. [Google Scholar]
- Li, A.; Kaur, P. FACS enrichment of human keratinocyte stem cells. Methods Mol. Biol 2005, 289, 87–96. [Google Scholar]
- Marconi, A.; Dallaglio, K.; Lotti, R.; Vaschieri, C.; Truzzi, F.; Fantini, F.; Pincelli, C. Survivin identifies keratinocyte stem cells and is downregulated by anti-beta1 integrin during anoikis. Stem Cells 2007, 25, 149–155. [Google Scholar]
- Wicha, M.A.; Liu, S.; Dontu, G. A Paradigm Shift—Cancer Stem Cells: An Old Idea, a paradigm shift. Cancer Res 2006, 66, 1883–1890. [Google Scholar]
- Welte, Y.; Adjaye, J.; Lehrach, H.R.; Regenbrecht, C.R. Cancer stem cells in solid tumors: Elusive or illusive? Cell Commun. Signal 2010, 8, 6. [Google Scholar]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med 1997, 3, 730–737. [Google Scholar]
- Colmont, C.S.; Harding, K.G.; Piguet, V.; Patel, G.K. Human skin cancer stem cells: A tale of mice and men. Exp. Dermatol 2012, 21, 576–580. [Google Scholar]
- Driessens, G.; Beck, B.; Caauwe, A.; Simons, B.D.; Blanpain, C. Defining the mode of tumour growth by clonal analysis. Nature 2012, 488, 527–530. [Google Scholar]
- Schober, M.; Fuchs, E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-β and integrin/focal adhesion kinase (FAK) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 10544–10549. [Google Scholar]
- Ridgway, R.A.; Serrels, B.; Mason, S.; Kinnaird, A.; Muir, M.; Patel, H.; Muller, W.J.; Sansom, O.J.; Brunton, V.G. Focal adhesion kinase is required for β-catenin-induced mobilization of epidermal stem cells. Carcinogenesis 2012, 33, 2369–2376. [Google Scholar]
- Janes, S.M.; Watt, F.M. Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J. Cell Biol 2004, 166, 419–431. [Google Scholar]
- Jensen, K.B.; Jones, J.; Watt, F.M. A stem cell gene expression profile of human squamous cell carcinomas. Cancer Lett 2008, 272, 23–31. [Google Scholar]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells-perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006, 66, 9339–9344. [Google Scholar]
- Rheinwald, J.G.; Beckett, M.A. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell 1980, 22, 629–632. [Google Scholar]
- Purdie, K.J.; Pourreyron, C.; South, A.P. Isolation and culture of squamous cell carcinoma lines. Methods Mol. Biol 2011, 731, 151–159. [Google Scholar]
- Janes, S.M.; Watt, F.M. New roles for integrins in squamous-cell carcinoma. Nat. Rev. Cancer 2006, 6, 175–183. [Google Scholar]
- Larjava, H.; Peltonen, J.; Akiyama, S.K.; Yamada, S.S.; Gralnick, H.R.; Uitto, J.; Yamada, K.M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J. Cell Biol 1990, 110, 803–815. [Google Scholar]
- Taylor, G.; Lehrer, M.S.; Jensen, P.J.; Sun, T.T.; Lavker, R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000, 102, 451–461. [Google Scholar]
- Pincelli, C.; Marconi, A. Keratinocyte stem cells: Friends and foes. J. Cell Physiol 2010, 225, 310–315. [Google Scholar] [Green Version]
- Dallaglio, K.; Marconi, A.; Truzzi, F.; Lotti, R.; Palazzo, E.; Petrachi, T.; Saltari, A.; Coppini, M.; Pincelli, C. E-FABP induces differentiation in normal human keratinocytes and modulates the differentiation process in psoriatic keratinocytes in vitro. Exp. Dermatol 2013, 22, 255–261. [Google Scholar]
- Masouyé, I.; Saurat, J.H.; Siegenthaler, G. Epidermal fatty-acid-binding protein in psoriasis, basal and squamous cell carcinomas: An immunohistological study. Dermatology 1996, 192, 208–213. [Google Scholar]
- Feng, R.; Zhou, S.; Liu, Y.; Song, D.; Luan, Z.; Dai, X.; Li, Y.; Tang, N.; Wen, J.; Li, L. Sox2 protects neural stem cells from apoptosis via up-regulating survivin expression. Biochem. J 2013, 450, 459–468. [Google Scholar]
- Carter, B.Z.; Qiu, Y.; Huang, X.; Diao, L.; Zhang, N.; Coombes, K.R.; Mak, D.H.; Konopleva, M.; Cortes, J.; Kantarjian, H.M.; et al. Survivin is highly expressed in CD34(+)38(−) leukemic stem/progenitor cells and predicts poor clinical outcomes in AML. Blood 2012, 120, 173–180. [Google Scholar]
- Reichert, S.; Rodel, C.; Mirsch, J.; Harter, P.N.; Tomicic, M.T.; Mittelbronn, M.; Kaina, B.; Rödel, F. Survivin inhibition and DNA double-strand break repair: A molecular mechanism to overcome radioresistance in glioblastoma. Radiother. Oncol 2011, 101, 51–58. [Google Scholar]
- Acquati, S.; Greco, A.; Licastro, D.; Bhagat, H.; Ceric, D.; Rossini, Z.; Grieve, J.; Shaked-Rabi, M.; Henriquez, N.V.; Brandner, S.; et al. Epigenetic regulation of survivin by Bmi1 is cell type specific during corticogenesis and in gliomas. Stem Cells 2013, 31, 190–202. [Google Scholar]
- Beck, B.; Driessens, G.; Goossens, S.; Youssef, K.K.; Kuchnio, A.; Caauwe, A.; Sotiropoulou, P.A.; Loges, S.; Lapouge, G.; Candi, A.; et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 2011, 478, 399–403. [Google Scholar]
- Ghadially, R. 25 years of epidermal stem cell research. J. Invest. Dermatol 2012, 132, 797–810. [Google Scholar]
- Patel, G.K.; Yee, C.L.; Terunuma, A.; Telford, W.G.; Voong, N.; Yuspa, S.H.; Vogel, J.C. Identification and characterization of tumor-initiating cells in human primary cutaneous squamous cell carcinoma. J. Invest. Dermatol 2012, 132, 401–409. [Google Scholar]
- Sun, Y.; Kong, W.; Falk, A.; Hu, J.; Zhou, L.; Pollard, S.; Smith, A. CD133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS One 2009, 4, e5498. [Google Scholar]
- Jeong, H.W.; Kim, I.S. TGF-beta1 enhances betaig-h3-mediated keratinocyte cell migration through the alpha3beta1 integrin and PI3K. J. Cell. Biochem 2004, 92, 770–780. [Google Scholar]
- Reuter, J.A.; Ortiz-Urda, S.; Kretz, M.; Garcia, J.; Scholl, F.A.; Pasmooij, A.M.; Cassarino, D.; Chang, H.Y.; Khavari, P.A. Modeling inducible human tissue neoplasia identifies an extracellular matrix interaction network involved in cancer progression. Cancer Cell 2009, 15, 477–488. [Google Scholar]
- Ferreira, M.; Fujiwara, H.; Morita, K.; Watt, F.M. An activating beta1 integrin mutation increases the conversion of benign to malignant skin tumors. Cancer Res 2009, 15, 1334–1342. [Google Scholar]
- Dallaglio, K.; Marconi, A.; Pincelli, C. Survivin: A dual player in healthy and diseased skin. J. Invest. Dermatol 2012, 132, 18–27. [Google Scholar]
- Altieri, D.C. Targeting survivin in cancer. Cancer Lett 2013, 332, 225–228. [Google Scholar]
- Gandarillas, A.; Watt, F.M. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev 1997, 11, 2869–2882. [Google Scholar]
- Worst, P.K.; Mackenzie, I.C.; Fusenig, N.E. Reformation of organized epidermal structure by transplantation of suspensions and cultures of epidermal and dermal cells. Cell Tissue Res 1982, 225, 65–77. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dallaglio, K.; Petrachi, T.; Marconi, A.; Truzzi, F.; Lotti, R.; Saltari, A.; Morandi, P.; Puviani, M.; Maiorana, A.; Roop, D.R.; et al. Isolation and Characterization of Squamous Cell Carcinoma-Derived Stem-like Cells: Role in Tumor Formation. Int. J. Mol. Sci. 2013, 14, 19540-19555. https://doi.org/10.3390/ijms141019540
Dallaglio K, Petrachi T, Marconi A, Truzzi F, Lotti R, Saltari A, Morandi P, Puviani M, Maiorana A, Roop DR, et al. Isolation and Characterization of Squamous Cell Carcinoma-Derived Stem-like Cells: Role in Tumor Formation. International Journal of Molecular Sciences. 2013; 14(10):19540-19555. https://doi.org/10.3390/ijms141019540
Chicago/Turabian StyleDallaglio, Katiuscia, Tiziana Petrachi, Alessandra Marconi, Francesca Truzzi, Roberta Lotti, Annalisa Saltari, Paolo Morandi, Mario Puviani, Antonino Maiorana, Dennis R. Roop, and et al. 2013. "Isolation and Characterization of Squamous Cell Carcinoma-Derived Stem-like Cells: Role in Tumor Formation" International Journal of Molecular Sciences 14, no. 10: 19540-19555. https://doi.org/10.3390/ijms141019540




