Erucin Exerts Anti-Inflammatory Properties in Murine Macrophages and Mouse Skin: Possible Mediation through the Inhibition of NFκB Signaling
Abstract
:1. Introduction
2. Results and Discussion
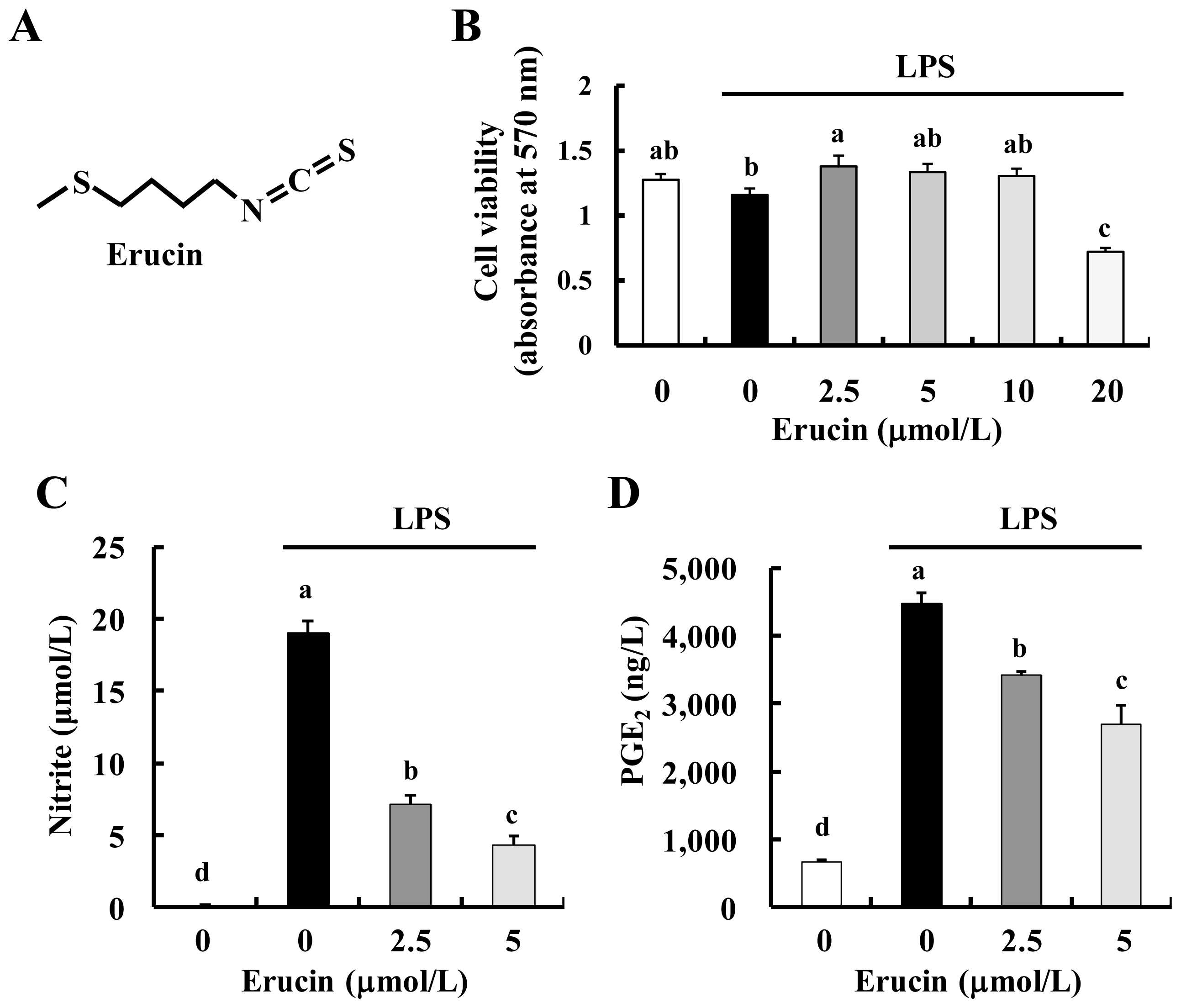
2.1. Erucin Decreases LPS-Induced Production of NO and PGE2 in RAW 264.7 Cells
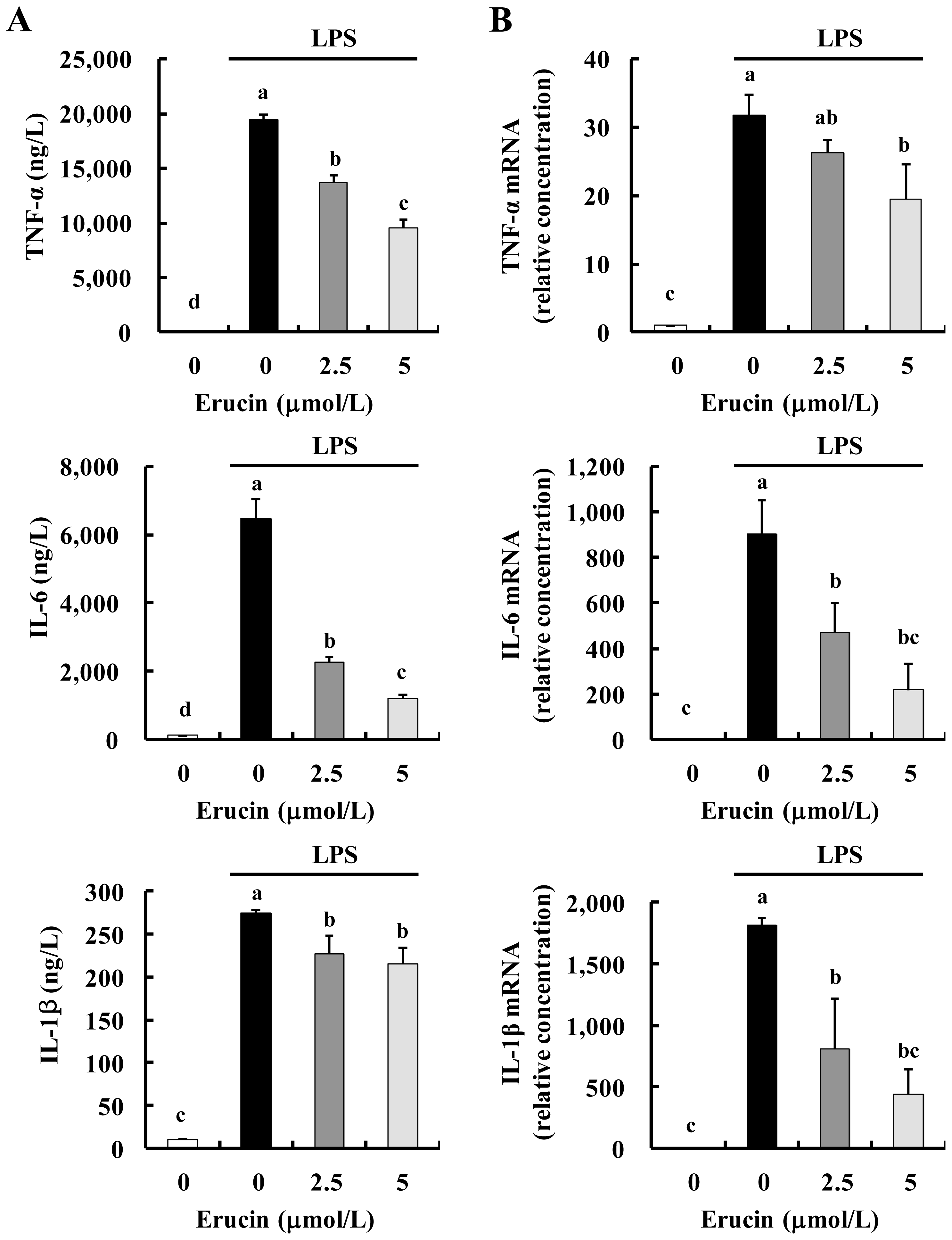
2.2. Erucin Decreases LPS-Induced Production of TNF-α, IL-6 and IL-1β in RAW 264.7 Cells
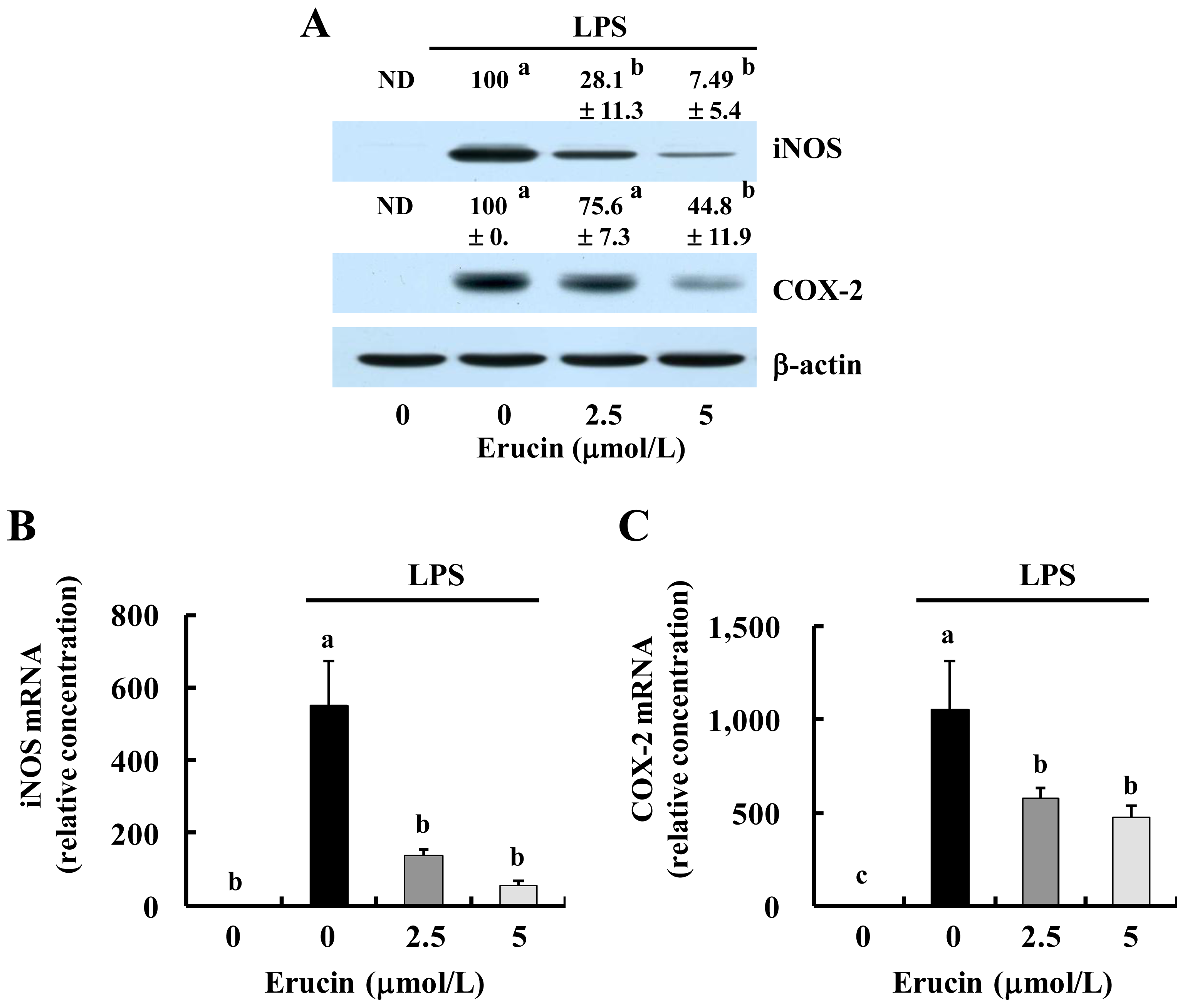
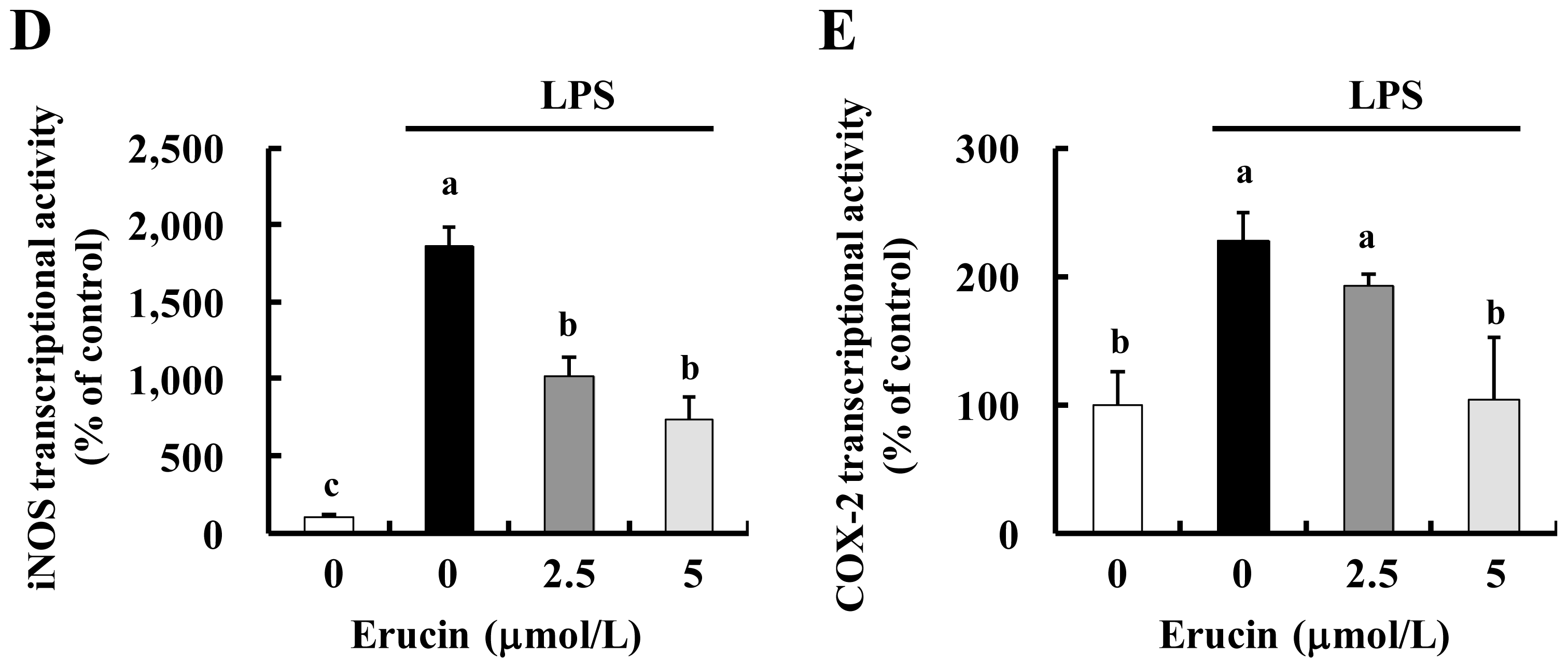
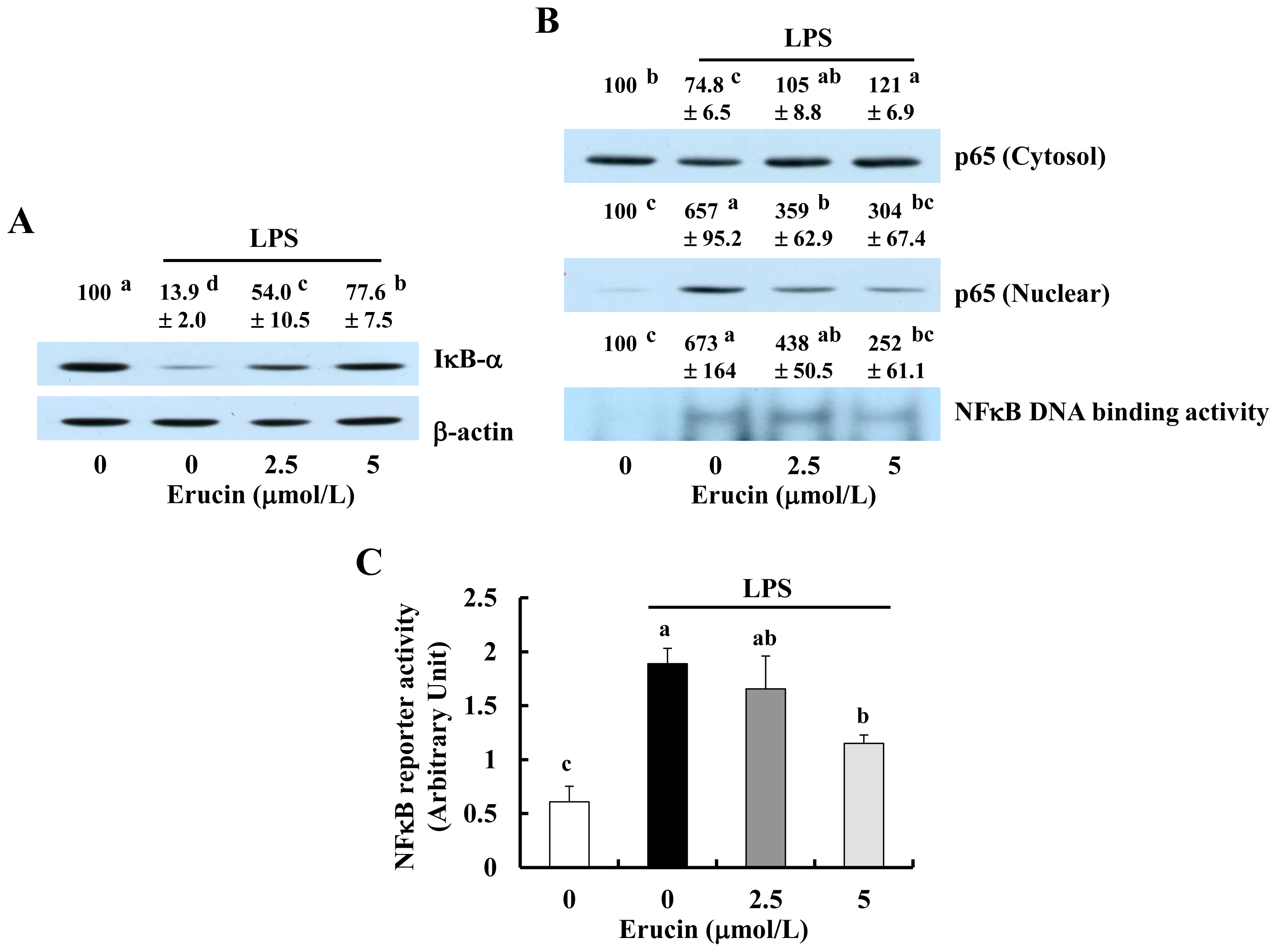
2.3. Erucin Inhibits LPS-Induced Activation of NFκB Signaling in RAW 264.7 Cells
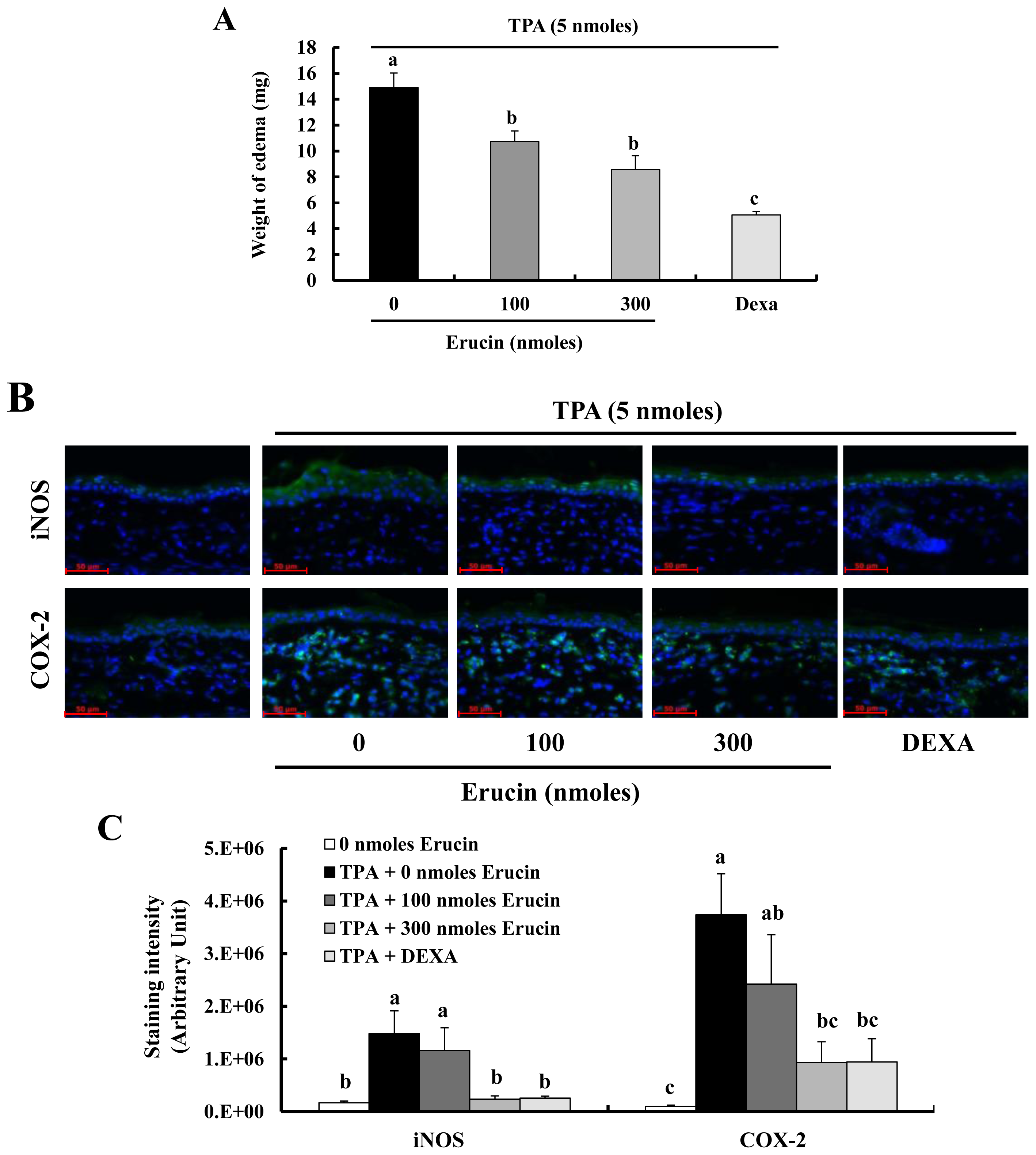
2.4. Erucin Decreases TPA-Induced Edema Formation in a Mouse Inflammation Model
2.5. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Culture and MTT Assay
3.3. NO, PGE2 and Cytokine Assays
3.4. Western Blot Analysis and Electrophoretic Mobility Shift Assay (EMSA)
3.5. Real-Time RT-PCR
3.6. Luciferase Reporter Gene Assay
3.7. Mouse Ear Edema
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Aller, M.A.; Arias, N.; Fuentes-Julian, S.; Blazquez-Martinez, A.; Argudo, S.; Miguel, M.P.; Arias, J.L.; Arias, J. Coupling inflammation with evo-devo. Med. Hypotheses 2012, 78, 721–731. [Google Scholar]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest 2007, 117, 1175–1183. [Google Scholar]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res 2006, 4, 221–233. [Google Scholar]
- Manchali, S.; Chidambara Murthy, K.N.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res 2007, 55, 224–236. [Google Scholar]
- Brown, K.K.; Hampton, M.B. Biological targets of isothiocyanates. Biochim. Biophys. Acta 2011, 1810, 888–894. [Google Scholar]
- Lee, Y.M.; Seon, M.R.; Cho, H.J.; Kim, J.S.; Park, J.H. Benzyl isothiocyanate exhibits anti-inflammatory effects in murine macrophages and in mouse skin. J. Mol. Med 2009, 87, 1251–1261. [Google Scholar]
- Lee, Y.M.; Cho, H.J.; Ponnuraj, S.P.; Kim, J.; Kim, J.S.; Kim, S.G.; Park, J.H. Phenethyl isothiocyanate inhibits 12-O-tetradecanoylphorbol-13-acetate-induced inflammatory responses in mouse skin. J. Med. Food 2011, 14, 377–385. [Google Scholar]
- Woo, K.J.; Kwon, T.K. Sulforaphane suppresses lipopolysaccharide-induced cyclooxygenase-2 (COX-2) expression through the modulation of multiple targets in COX-2 gene promoter. Int. Immunopharmacol 2007, 7, 1776–1783. [Google Scholar]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhauser, C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem 2001, 276, 32008–32015. [Google Scholar]
- Melchini, A.; Traka, M.H. Biological profile of erucin: A new promising anticancer agent from cruciferous vegetables. Toxins 2010, 2, 593–612. [Google Scholar]
- Gasparini, C.; Feldmann, M. NF-kappaB as a target for modulating inflammatory responses. Curr. Pharm. Des 2012, 18, 5735–5745. [Google Scholar]
- Valledor, A.F.; Comalada, M.; Santamaria-Babi, L.F.; Lloberas, J.; Celada, A. Macrophage proinflammatory activation and deactivation: A question of balance. Adv. Immunol 2010, 108, 1–20. [Google Scholar]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol 2012, 188, 21–28. [Google Scholar]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: Key regulators of the NF-kappaB pathway. Trends Biochem. Sci 2004, 29, 72–79. [Google Scholar]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Invest 2001, 107, 135–142. [Google Scholar]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol 2009, 1, a001651. [Google Scholar]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov 2009, 8, 33–40. [Google Scholar]
- Cho, H.J.; Lim, S.S.; Lee, Y.S.; Kim, J.S.; Lee, C.H.; Kwon, D.Y.; Park, J.H. Hexane/ethanol extract of Glycyrrhiza uralensis licorice exerts potent anti-inflammatory effects in murine macrophages and in mouse skin. Food Chem 2010, 121, 959–966. [Google Scholar]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr 2008, 47, 73–88. [Google Scholar]
- Rao, C.V. Benzyl isothiocyanate: Double trouble for breast cancer cells. Cancer Prev. Res 2013, 6, 760–763. [Google Scholar]
- Cheung, K.L.; Kong, A.N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J 2010, 12, 87–97. [Google Scholar]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar]
- Yehuda, H.; Soroka, Y.; Zlotkin-Frusic, M.; Gilhar, A.; Milner, Y.; Tamir, S. Isothiocyanates inhibit psoriasis-related proinflammatory factors in human skin. Inflamm. Res 2012, 61, 735–742. [Google Scholar]
- Abbaoui, B.; Riedl, K.M.; Ralston, R.A.; Thomas-Ahner, J.M.; Schwartz, S.J.; Clinton, S.K.; Mortazavi, A. Inhibition of bladder cancer by broccoli isothiocyanates sulforaphane and erucin: Characterization, metabolism, and interconversion. Mol. Nutr. Food Res 2012, 56, 1675–1687. [Google Scholar]
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res 2011, 64, 456–463. [Google Scholar]
- Vermeulen, M.; Van den Berg, R.; Freidig, A.P.; van Bladeren, P.J.; Vaes, W.H. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. J. Agric. Food Chem 2006, 54, 5350–5358. [Google Scholar]
- Cho, H.; Seon, M.; Lee, Y.; Kim, J.; Kim, J.; Kim, S.; Park, J. 3,3′-Diindolylmethane suppresses the inflammatory response to lipopolysaccharide in murine macrophages. J. Nutr 2008, 138, 17–23. [Google Scholar]
- Cho, I.J.; Lee, A.K.; Lee, S.J.; Lee, M.G.; Kim, S.G. Repression by oxidative stress of iNOS and cytokine gene induction in macrophages results from AP-1 and NF-kappaB inhibition mediated by B cell translocation gene-1 activation. Free Radic. Biol. Med 2005, 39, 1523–1536. [Google Scholar]
- Ki, S.H.; Choi, M.J.; Lee, C.H.; Kim, S.G. Galpha12 specifically regulates COX-2 induction by sphingosine 1-phosphate. Role for JNK-dependent ubiquitination and degradation of IkappaBalpha. J. Biol. Chem 2007, 282, 1938–1947. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cho, H.J.; Lee, K.W.; Park, J.H.Y. Erucin Exerts Anti-Inflammatory Properties in Murine Macrophages and Mouse Skin: Possible Mediation through the Inhibition of NFκB Signaling. Int. J. Mol. Sci. 2013, 14, 20564-20577. https://doi.org/10.3390/ijms141020564
Cho HJ, Lee KW, Park JHY. Erucin Exerts Anti-Inflammatory Properties in Murine Macrophages and Mouse Skin: Possible Mediation through the Inhibition of NFκB Signaling. International Journal of Molecular Sciences. 2013; 14(10):20564-20577. https://doi.org/10.3390/ijms141020564
Chicago/Turabian StyleCho, Han Jin, Ki Won Lee, and Jung Han Yoon Park. 2013. "Erucin Exerts Anti-Inflammatory Properties in Murine Macrophages and Mouse Skin: Possible Mediation through the Inhibition of NFκB Signaling" International Journal of Molecular Sciences 14, no. 10: 20564-20577. https://doi.org/10.3390/ijms141020564




