Association of Metabolic Syndrome and Albuminuria with Cardiovascular Risk in Occupational Drivers
Abstract
:Background and Aim
Methods
Results
Conclusions
1. Introduction
2. Results
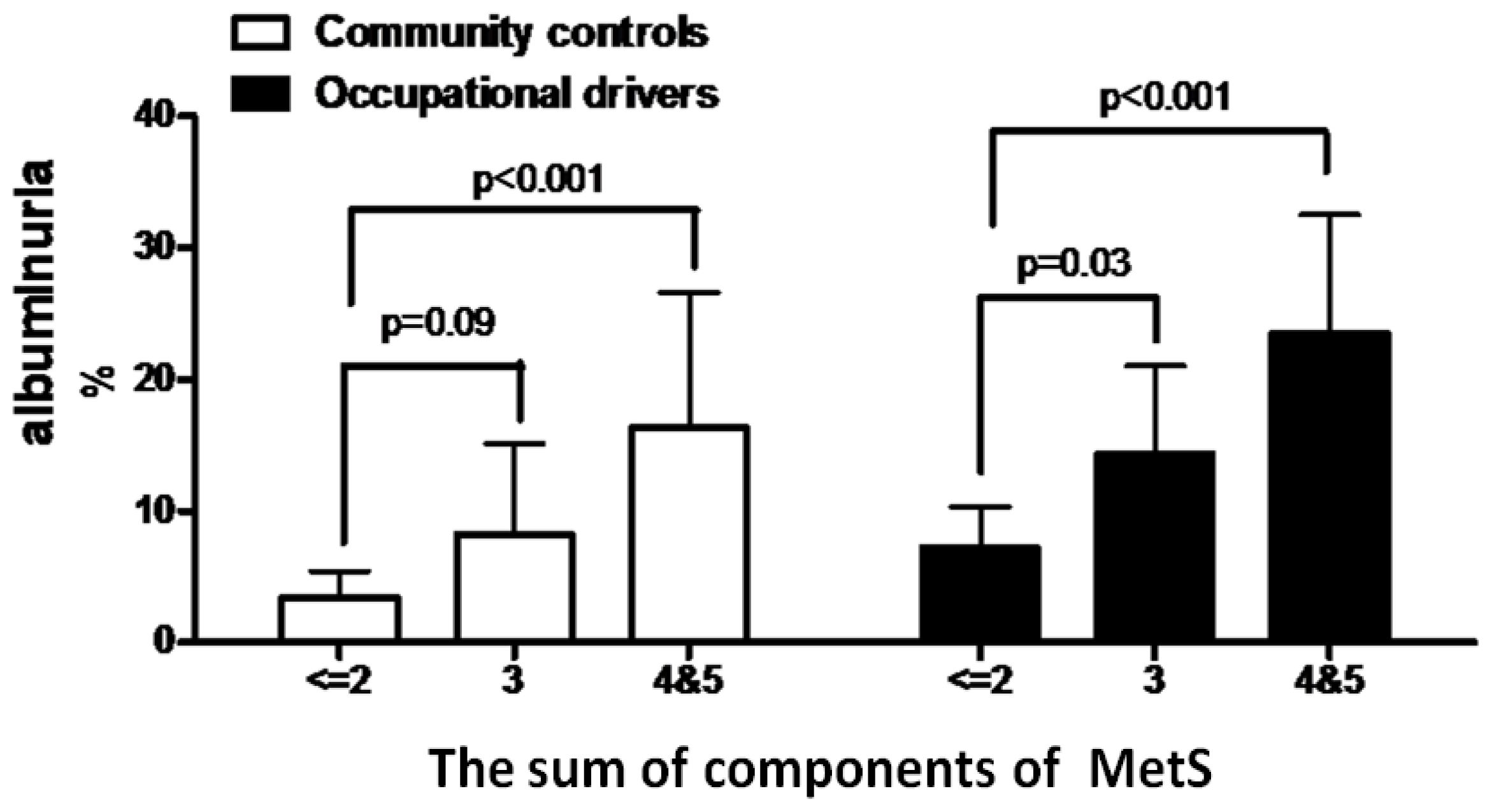
2.1. Determinants of MetS in All Subjects
2.2. Determinants of Albuminuria in All Subjects
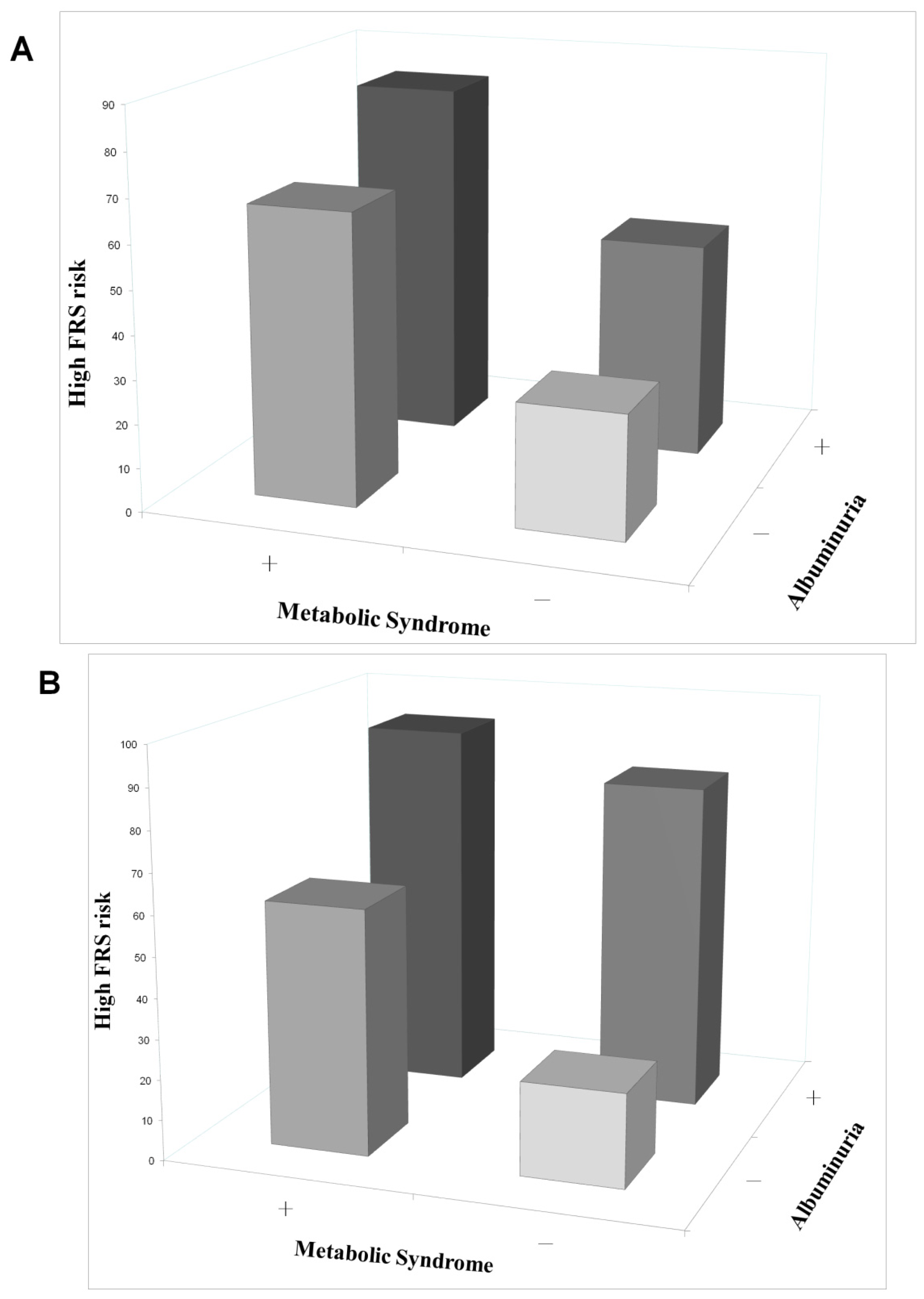
2.3. Determinants of MetS and Albuminuria in Occupational Drivers
3. Discussion
4. Subjects and Methods
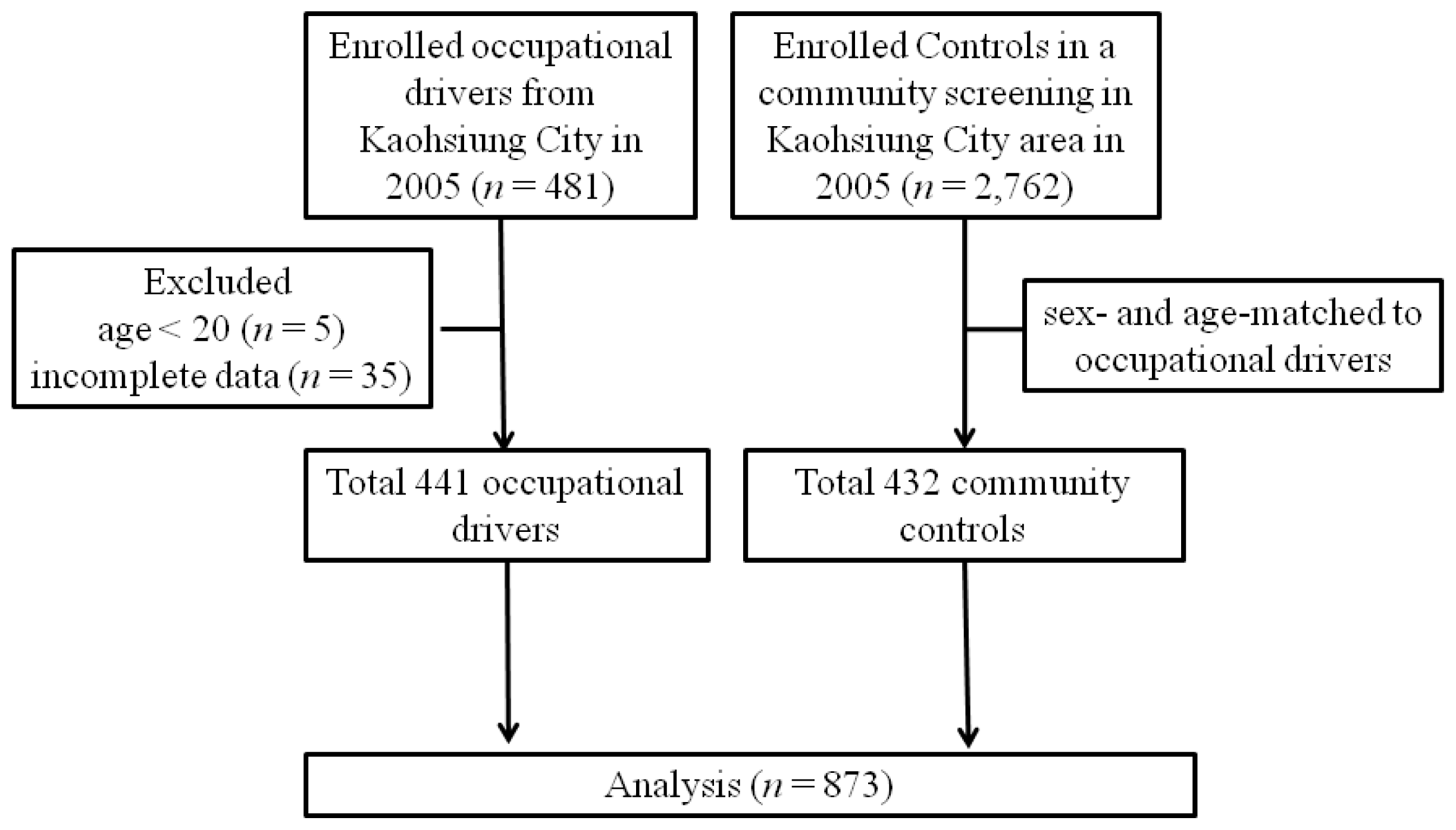
4.1. Study Patients and Design
4.2. Laboratory Investigations
4.3. Definition of MetS and Albuminuria
4.4. FRS Calculation and Risk Category
4.5. Questionnaire
4.6. Statistical Analysis
5. Conclusions
Figures and Tables
| Variables | Occupational drivers (n = 441) | Community controls (n = 432) | p value |
|---|---|---|---|
| Age (year) | 46.5 ± 9.4 | 47.5 ± 13.6 | 0.21 |
| Male gender (%) | 96.8 | 95.8 | 0.44 |
| Education (year) | |||
| ≤6 | 17.5 | 11.1 | <0.001 |
| 7–12 | 73.7 | 20.1 | – |
| ≥12 | 8.8 | 63.4 | – |
| Unknown | 0 | 5.4 | – |
| Driving time (h/days) | |||
| ≤8 | 38.3 | – | – |
| 9–12 | 50.3 | – | – |
| ≥13 | 11.3 | – | – |
| Co-morbidities | |||
| Renal diseases (%) | 4.8 | 3.2 | 0.32 |
| Diabetes mellitus (%) | 4.3 | 6.7 | 0.08 |
| Hypertension (%) | 10.0 | 15.0 | 0.01 |
| Liver diseases (%) | 4.3 | 5.1 | 0.48 |
| Cardiovascular disease (%) | 2.7 | 4.9 | 0.07 |
| Dyslipidemia (%) | 4.1 | 6.3 | 0.11 |
| Gout (%) | 6.6 | 5.3 | 0.55 |
| Habits | |||
| Smoking (current) (%) | 47.2 | 0 | <0.001 |
| Drinking (ever) (%) | 71.2 | 41.0 | <0.001 |
| Betel nut chewing (ever) (%) | 50.6 | 6.5 | <0.001 |
| Exercise (30 min/time, 3 times/week) (%) | 24.7 | 46.1 | <0.001 |
| Systolic BP (mmHg) | 133.0 ± 19.3 | 125.9 ± 16.6 | <0.001 |
| Diastolic BP (mmHg) | 82.8 ± 12.6 | 79.2 ± 10.8 | <0.001 |
| Body mass index (kg/m2) | 26.2 ± 3.6 | 24.5 ± 3.3 | <0.001 |
| Waist circumference (cm) | 88.7 ± 10.3 | 85.7 ± 9.1 | <0.001 |
| Laboratory parameters | |||
| Glucose (mg/dL) | 105.4 ± 47.3 | 92.1 ± 28.1 | <0.001 |
| Total cholesterol (mg/dL) | 211.4 ± 47.5 | 201.6 ± 37.2 | 0.01 |
| HDL-cholesterol (mg/dL) | 53.2 ± 13.6 | 55.3 ± 13.6 | 0.02 |
| LDL-cholesterol (mg/dL) | 133.3 ± 39.2 | 132.0 ± 33.9 | 0.65 |
| Triglyceride (mg/dL) | 152 (105.25–229.1) | 118.55 (84.2–178.25) | <0.001 |
| Uric acid (mg/dL) | 6.8 ± 1.5 | 6.7 ± 1.4 | 0.29 |
| eGFR (mL/min/1.73 m2) | 80.6 ± 15.6 | 79.9 ± 14.9 | 0.49 |
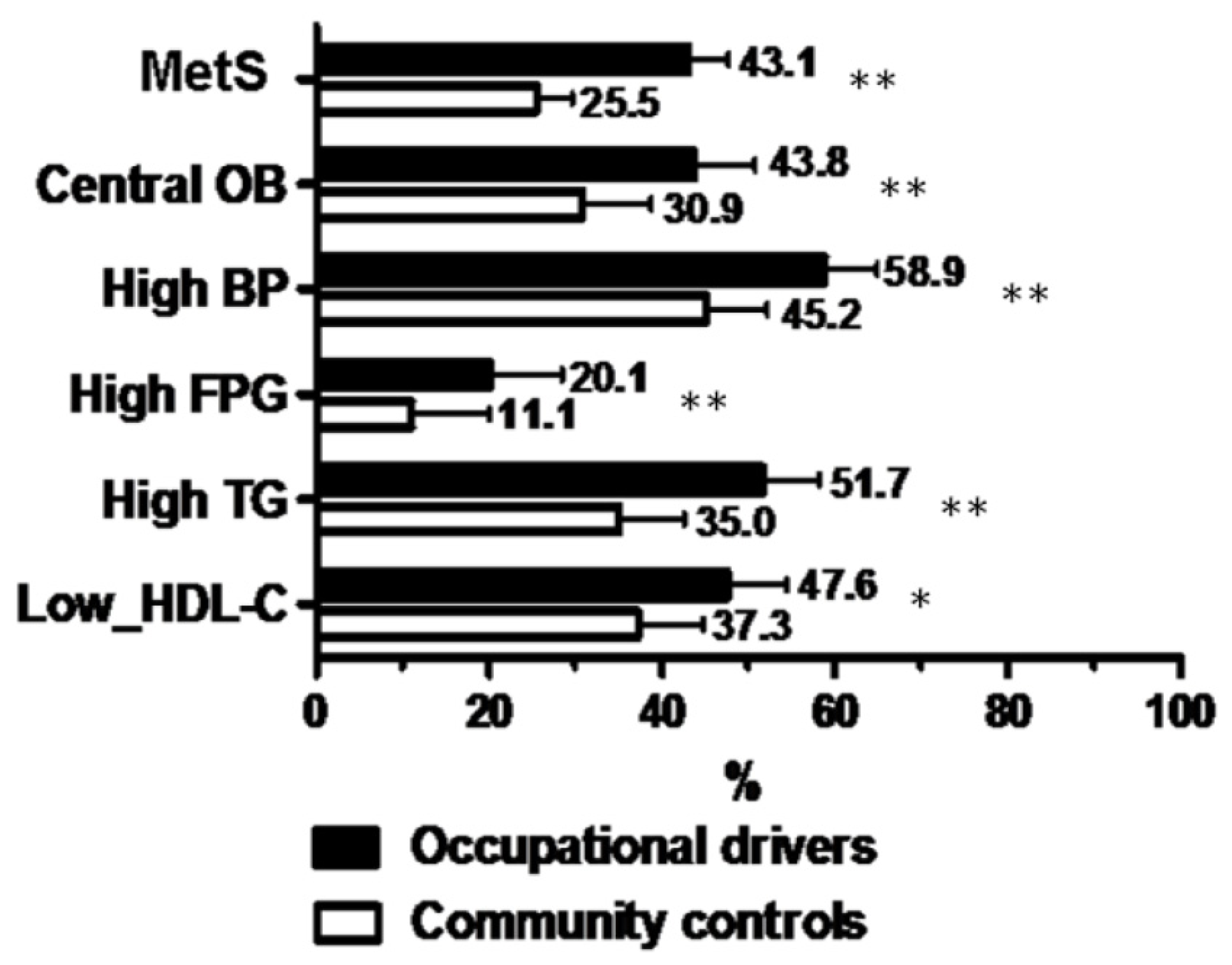
| MetS (%) | 43.1 | 25.5 | <0.001 |
| Albuminuria (%) | 12.0 | 5.6 | 0.01 |
| High FRS risk (%) | 46.9 | 35.2 | <0.001 |
| Parameter | Univariate | Multivariate (Forward) | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Occupational drivers vs. Community controls | 2.22 (1.66–2.95) | <0.001 | 1.70 (1.20–2.42) | 0.01 |
| Age (per 1 year) | 1.02 (1.01–1.04) | <0.001 | 1.03 (1.01–1.04) | <0.001 |
| Male gender | 5.28 (1.59–17.47) | 0.01 | 4.92 (1.43–16.96) | 0.01 |
| Driving time (h/days) | ||||
| ≤8 | Reference | – | – | – |
| 9–12 | 1.01 (0.67–1.51) | 0.97 | – | – |
| ≥13 | 1.15 (0.61–2.16) | 0.67 | – | – |
| Co-morbidities | ||||
| Renal diseases | 1.84 (0.93–3.62) | 0.08 | – | – |
| Diabetes mellitus | 2.82 (1.56–5.10) | 0.01 | 2.60 (1.35–5.00) | 0.01 |
| Hypertension | 1.45 (0.96–2.19) | 0.08 | – | – |
| Liver diseases | 0.77 (0.39–1.54) | 0.46 | – | – |
| Cardiovascular disease | 0.82 (0.38–1.74) | 0.60 | – | – |
| Dyslipidemia | 1.41 (0.77–2.59) | 0.27 | – | – |
| Gout | 3.00 (1.69–5.33) | <0.001 | 2.31 (1.26–4.23) | 0.01 |
| Habits | ||||
| Smoking (current vs. former) | 2.07 (1.50–2.85) | <0.001 | – | – |
| Drinking (ever vs. never) | 1.36 (1.02–1.81) | 0.04 | – | – |
| Betel nut chewing (ever vs. never) | 2.39 (1.76–3.23) | <0.001 | 2.03 (1.41–2.92) | <0.001 |
| Exercise (30 min/time, 3 times/week) | 0.75 (0.56–1.01) | 0.06 | – | – |
| Albuminuria | 3.57 (2.20–5.80) | <0.001 | 2.75 (1.63–4.65) | <0.001 |
| Parameter | Univariate | Multivariate (Forward) | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Occupational drivers vs. Community controls | 2.32 (1.41–3.84) | 0.01 | 2.65 (1.51–4.87) | 0.01 |
| Age (per 1 year) | 1.05 (1.03–1.08) | <0.001 | 1.05 (1.02–1.08) | < 0.001 |
| Male gender | 0.51 (0.19–1.35) | 0.17 | – | – |
| Driving time (h/days) | ||||
| ≤8 | Reference | – | – | – |
| 9–12 | 0.77 (0.42–1.42) | 0.40 | – | – |
| ≥13 | 0.87 (0.33–2.26) | 0.77 | – | – |
| Co-morbidities | ||||
| Renal diseases | 3.88 (1.75–8.62) | 0.01 | 2.68 (1.14–6.30) | 0.02 |
| Diabetes mellitus | 3.39 (1.65–1.95) | 0.01 | – | – |
| Hypertension | 2.99 (1.73–5.18) | <0.001 | 2.40 (1.32–4.36) | 0.01 |
| Liver diseases | 1.11 (0.39–3.21) | 0.85 | – | – |
| Cardiovascular disease | 1.88 (0.71–5.03) | 0.21 | – | – |
| Dyslipidemia | 1.62 (0.66–3.97) | 0.29 | – | – |
| Gout | 2.29 (1.07–4.91) | 0.03 | – | – |
| Habits | ||||
| Smoking (current vs. former) | 1.11 (0.65–1.90) | 0.70 | – | – |
| Drinking (ever vs. never) | 1.00 (0.63–1.61) | 0.99 | – | – |
| Betel nut chewing (ever vs. never) | 0.96 (0.57–1.62) | 0.89 | – | – |
| Exercise (30 min/time, 3 times/week) | 1.00 (0.61–1.63) | 0.99 | – | – |
| MetS | 3.57 (2.20–5.80) | <0.001 | 2.58 (1.56–4.29) | <0.001 |
| Parameter | Multivariate (Forward) | |
|---|---|---|
| MetS | Adjusted OR (95% CI) | p |
| Age (per 1 year) | 1.03 (1.00–1.05) | 0.03 |
| Diabetes mellitus | 6.29 (1.64–24.04) | 0.01 |
| Gout | 3.25 (1.35–7.82) | 0.03 |
| Betel nut chewing (ever vs. never) | 2.06 (1.36–3.14) | 0.01 |
| Exercise (30 min/time, 3 times/week) | 0.55 (0.34–0.90) | 0.02 |
| Albuminuria | 2.75 (1.41–5.37) | 0.01 |
| Albuminuria | Adjusted OR (95% CI) | p |
| Renal diseases | 4.16 (1.48–11.73) | 0.01 |
| Diabetes mellitus | 4.98 (1.79–13.87) | 0.01 |
| Hypertension | 3.66 (1.69–7.92) | 0.01 |
| MetS | 2.28 (1.20–4.30) | 0.01 |
Conflicts of Interest
References
- Ford, E.S. The metabolic syndrome and mortality from cardiovascular disease and all-causes: Findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis 2004, 173, 309–314. [Google Scholar]
- Chen, B.; Yang, D.; Chen, Y.; Xu, W.; Ye, B.; Ni, Z. The prevalence of microalbuminuria and its relationships with the components of metabolic syndrome in the general population of China. Clin. Chim. Acta 2010, 411, 705–709. [Google Scholar]
- Danziger, J. Importance of low-grade albuminuria. Mayo Clin. Proc 2008, 83, 806–812. [Google Scholar]
- Abdelhafiz, A.H.; Ahmed, S.; El Nahas, M. Microalbuminuria: Marker or maker of cardiovascular disease. Nephron. Exp. Nephrol 2011, 119, e6–e10. [Google Scholar]
- National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421.
- Lucove, J.; Vupputuri, S.; Heiss, G.; North, K.; Russell, M. Metabolic syndrome and the development of CKD in American Indians: The Strong Heart Study. Am. J. Kidney Dis 2008, 51, 21–28. [Google Scholar]
- Tozawa, M.; Iseki, C.; Tokashiki, K.; Chinen, S.; Kohagura, K.; Kinjo, K.; Takishita, S.; Iseki, K. Metabolic syndrome and risk of developing chronic kidney disease in Japanese adults. Hypertens Res 2007, 30, 937–943. [Google Scholar]
- Mohebbi, I.; Saadat, S.; Aghassi, M.; Shekari, M.; Matinkhah, M.; Sehat, S. Prevalence of metabolic syndrome in Iranian professional drivers: Results from a population based study of 12,138 men. PLoS One 2012, 7, e31790. [Google Scholar]
- Medical Fitness to Drive in the Transportation Industry and the Impact on Public Safety: Recommended Actions; American College of Occupational and Environmental Medicine: Elk Grove Village, IL, USA, 2012.
- Anderson, J.E.; Govada, M.; Steffen, T.K.; Thorne, C.P.; Varvarigou, V.; Kales, S.N.; Burks, S.V. Obesity is associated with the future risk of heavy truck crashes among newly recruited commercial drivers. Accid. Anal. Prev 2012, 49, 378–384. [Google Scholar]
- Siu, S.C.; Wong, K.W.; Lee, K.F.; Lo, Y.Y.; Wong, C.K.; Chan, A.K.; Fong, D.Y.; Lam, C.L. Prevalence of undiagnosed diabetes mellitus and cardiovascular risk factors in Hong Kong professional drivers. Diabetes Res. Clin. Pract 2012, 96, 60–67. [Google Scholar]
- Spaggiari, M.C. Circadian sleep-wake disorders and professional driving. G Ital. Med. Lav. Ergon 2012, 34, 329–332. [Google Scholar]
- Akkoyunlu, M.E.; Altın, R.; Kart, L.; Atalay, F.; Ornek, T.; Bayram, M.; Tor, M. Investigation of obstructive sleep apnoea syndrome prevalence among long-distance drivers from Zonguldak, Turkey. Multidiscip. Respir. Med 2013. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Dev, S. Effect of low back pain on social and professional life of drivers of Kolkata. Work 2012, 41, 2426–2433. [Google Scholar]
- Luo, W.W.; Zhao, J.; Fu, Y.X.; Ma, A.H. The clinical analysis of 51 taxi drivers with peptic ulcer. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2012, 30, 131–132. [Google Scholar]
- De Bacquer, D.; van Risseghem, M.; Clays, E.; Kittel, F.; de Backer, G.; Braeckman, L. Rotating shift work and the metabolic syndrome: A prospective study. Int. J. Epidemiol 2009, 38, 848–854. [Google Scholar]
- Cavagioni, L.C.; Bensenor, I.M.; Halpern, A.; Pierin, A.M. Metabolic Syndrome in professional truck drivers who work on Highway BR-116 within the area of Sao Paulo City—Regis Bittencourt. Arq. Bras. Endocrinol. Metabol 2008, 52, 1015–1023. [Google Scholar]
- Saberi, H.R.; Moravveji, A.R.; Fakharian, E.; Kashani, M.M.; Dehdashti, A.R. Prevalence of metabolic syndrome in bus and truck drivers in Kashan, Iran. Diabetol. Metab. Syndr 2011, 3, 8–13. [Google Scholar]
- Branth, S.; Ronquist, G.; Stridsberg, M.; Hambraeus, L.; Kindgren, E.; Olsson, R.; Carlander, D.; Arnetz, B. Development of abdominal fat and incipient metabolic syndrome in young healthy men exposed to long-term stress. Nutr. Metab. Cardiovasc. Dis 2007, 17, 427–435. [Google Scholar]
- Nakamura, K.; Shimai, S.; Kikuchi, S.; Takahashi, H.; Tanaka, M.; Nakano, S.; Motohashi, Y.; Nakadaira, H.; Yamamoto, M. Increases in body mass index and waist circumference as outcomes of working overtime. Occup. Med. (Lond.) 1998, 48, 169–173. [Google Scholar]
- Eberly, L.E.; Prineas, R.; Cohen, J.D.; Vazquez, G.; Zhi, X.; Neaton, J.D.; Kuller, L.H. Multiple Risk Factor Intervention Trial Research Group. Metabolic syndrome: Risk factor distribution and 18-year mortality in the multiple risk factor intervention trial. Diabetes Care 2006, 29, 123–130. [Google Scholar]
- Malik, A.R.; Sultan, S.; Turner, S.T.; Kullo, I.J. Urinary albumin excretion is associated with impaired flow- and nitroglycerin-mediated brachial artery dilatation in hypertensive adults. J. Hum. Hypertens 2007, 21, 231–238. [Google Scholar]
- Lee, C.H.; Ko, A.M.; Warnakulasuriya, S.; Yin, B.L.; Sunarjo Zain, R.B.; Ibrahim, S.O.; Liu, Z.W.; Li, W.H.; Zhang, S.S.; et al. Intercountry prevalences and practices of betel-quid use in south, southeast and eastern Asia regions and associated oral preneoplastic disorders: An international collaborative study by Asian betel-quid consortium of south and east Asia. Int. J. Cancer 2011, 129, 1741–1751. [Google Scholar]
- Chu, N.S. Neurological aspects of areca and betel chewing. Addict. Biol 2002, 7, 111–114. [Google Scholar]
- Guh, J.Y.; Chuang, L.Y.; Chen, H.C. Betel-quid use is associated with the risk of the metabolic syndrome in adults. Am. J. Clin. Nutr 2006, 83, 1313–1320. [Google Scholar]
- Carr, D.B.; Utzschneider, K.M.; Hull, R.L.; Kodama, K.; Retzlaff, B.M.; Brunzell, J.D.; Shofer, J.B.; Fish, B.E.; Knopp, R.H.; Kahn, S.E. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 2004, 53, 2087–2094. [Google Scholar]
- Blasi, C.; Jeanrenaud, B. Insulin resistance syndrome: Defective GABA neuromodulation as a possible hereditary pathogenetic factor (the “GABA hypothesis”). Med. Hypotheses 1993, 40, 197–206. [Google Scholar]
- Vickery, S.; Stevens, P.E.; Dalton, R.N.; van Lente, F.; Lamb, E.J. Does the ID-MS traceable MDRD equation work and is it suitable for use with compensated Jaffe and enzymatic creatinine assays? Nephrol. Dial. Transplant 2006, 21, 2439–2445. [Google Scholar]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. for Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med 1999, 130, 461–470. [Google Scholar]
- Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). J. Am. Med. Assoc 2001, 285, 2486–2497.
- Tan, C.E.; Ma, S.; Wai, D.; Chew, S.K.; Tai, E.S. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004, 27, 1182–1186. [Google Scholar]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, S.-C.; Chang, J.-M.; Lin, M.-Y.; Hou, M.-L.; Tsai, J.-C.; Hwang, S.-J.; Chen, H.-C. Association of Metabolic Syndrome and Albuminuria with Cardiovascular Risk in Occupational Drivers. Int. J. Mol. Sci. 2013, 14, 21997-22010. https://doi.org/10.3390/ijms141121997
Chen S-C, Chang J-M, Lin M-Y, Hou M-L, Tsai J-C, Hwang S-J, Chen H-C. Association of Metabolic Syndrome and Albuminuria with Cardiovascular Risk in Occupational Drivers. International Journal of Molecular Sciences. 2013; 14(11):21997-22010. https://doi.org/10.3390/ijms141121997
Chicago/Turabian StyleChen, Szu-Chia, Jer-Ming Chang, Ming-Yen Lin, Meng-Ling Hou, Jer-Chia Tsai, Shang-Jyh Hwang, and Hung-Chun Chen. 2013. "Association of Metabolic Syndrome and Albuminuria with Cardiovascular Risk in Occupational Drivers" International Journal of Molecular Sciences 14, no. 11: 21997-22010. https://doi.org/10.3390/ijms141121997






