Triterpenoids from the Roots of Rhaphiolepis indica var. tashiroi and Their Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Inhibition of Superoxide Production Activities
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Inhibition of Superoxide Production Assay
3.4.1. Evaluation of O2•− Release by Human Neutrophils
3.4.2. Preparation of Human Neutrophils
3.4.3. Measurement of O2•− Generation
3.4.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Ohashi, H. Rosaceae. In Flora of Taiwan, 2nd ed; Editorial Committee of the Flora of Taiwan, Editorial Committee of the Flora of Taiwan, Department of Botany, National Taiwan University: Taipei, Taiwan, 1993; Volume 3, pp. 69–157. [Google Scholar]
- Lin, C.H.; Chang, H.S.; Liao, C.H.; Ou, T.H.; Chen, I.S.; Tsai, I.L. Anti-inflammatory biphenyls and dibenzofurans from Rhaphiolepis indica. J. Nat. Prod 2010, 73, 1628–1631. [Google Scholar]
- Begum, S.; Farhat, S.; Siddiqui, B.S. Triterpenoids from the leaves of Eucalyptus camaldulensis var obtusa. J. Nat. Prod 1997, 60, 20–23. [Google Scholar]
- Zeng, N.; Shen, Y.; Li, L.Z.; Jiao, W.H.; Gao, P.Y.; Song, S.J.; Chen, W.S.; Lin, H.W. Anti-inflammatory triterpenes from the leaves of Rosa laevigata. J. Nat. Prod 2011, 74, 732–738. [Google Scholar]
- Chiu, H.L.; Wu, J.H.; Tung, Y.T.; Lee, T.H.; Chien, S.C.; Kuo, Y.H. Triterpenoids and aromatics from Derris laxiflora. J. Nat. Prod 2008, 71, 1829–1832. [Google Scholar]
- Gaind, K.N.; Singla, A.K.; Boar, R.B.; Copsey, D.B. Triterpenoids and sterols of Kalanchoe spathulata. Phytochemistry 1976, 15, 1999–2000. [Google Scholar]
- Susidarti, R.A.; Rahmani, M.; Ismail, H.B.M.; Sukari, M.A.; Hin, T.Y.Y.; Lian, G.E.C.; Ali, A.M.; Kulip, J.; Waterman, P.G. A new coumarin and triterpenes from Malaysian Micromelum minutum. Nat. Prod. Res 2006, 20, 145–151. [Google Scholar]
- Mahato, S.B.; Kundu, A.P. 13C NMR Spectra of pentacyclic triterpenoids-a compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar]
- Zhao, M.; Zhang, S.; Fu, L.; Li, N.; Bai, J.; Sakai, J.; Wang, L.; Tang, W.; Hasegawa, T.; Ogura, H.; et al. Taraxasterane- and ursane-type triterpenes from Nerium oleander and their biological activities. J. Nat. Prod 2006, 69, 1164–1167. [Google Scholar]
- Reher, G.; Budesinsky, M. Triterpenoids from plants of the Sanguisorbeae. Phytochemistry 1992, 31, 3909–3914. [Google Scholar]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest 1968, 21, 77–89. [Google Scholar]
- English, D.; Andersen, B.R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J. Immunol. Methods 1974, 5, 249–252. [Google Scholar]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest 1973, 52, 741–744. [Google Scholar]
- Watanabe, K.; Widyastuti, S.M.; Nonaka, F. Two biphenyl compounds from Rhaphiolepsis umbellata as its phytoalexin. Agric. Biol. Chem 1990, 54, 1861–1862. [Google Scholar]
- Widyastuti, S.M.; Nonaka, F.; Watanabe, K.; Maruyama, E.; Sako, N. Accumulation and antifungal spectrum of 4′-methoxyaucuparin as a new phytoalexin in Rhaphiolepsis umbellata Makino. Ann. Phytopath. Soc. Jpn 1991, 57, 232–238. [Google Scholar]
- Widyastuti, S.M.; Nonaka, F.; Watanabe, K.; Maruyama, E.; Sako, N. Accumulation and antifungal spectrum of rhaphiolepsin as a second new phytoalexin in Rhaphiolepsis umbellata Makino. Ann. Phytopath. Soc. Jpn 1991, 57, 641–648. [Google Scholar]
- Nonaka, G.I.; Ezaki, E.; Hayashi, K.; Nishioka, I. Flavanol glucosides from rhubarb and Rhaphiolepis umbellata. Phytochemistry 1983, 22, 1659–1661. [Google Scholar]
- Ezaki-Furuichi, E.; Nonaka, G.I.; Nishioka, I.; Hayashi, K. Isolation and structures of procyanidins (condensed tannins) from Rhaphiolepis umbellata. Agric. Biol. Chem 1986, 50, 2061–2067. [Google Scholar]
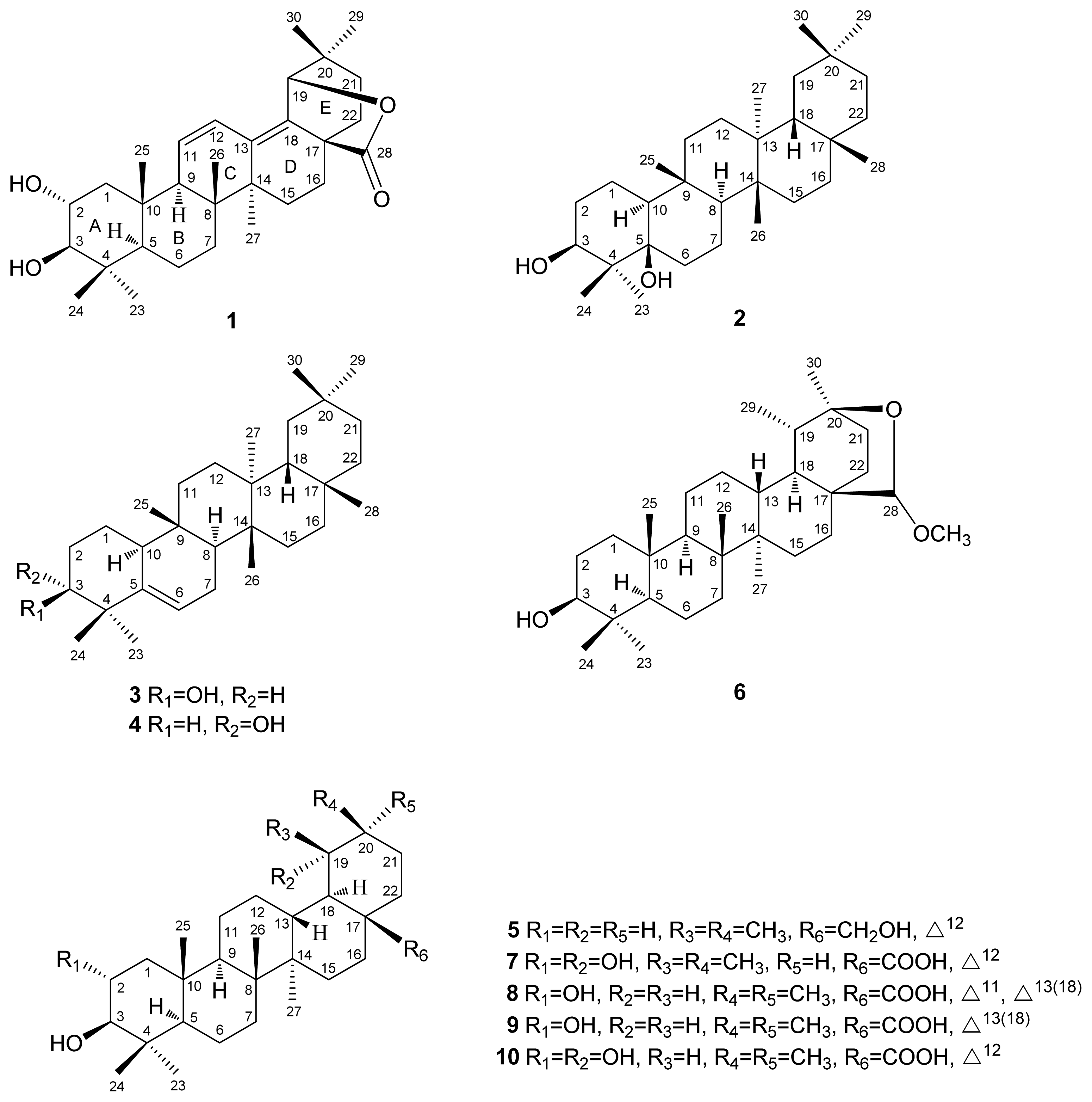

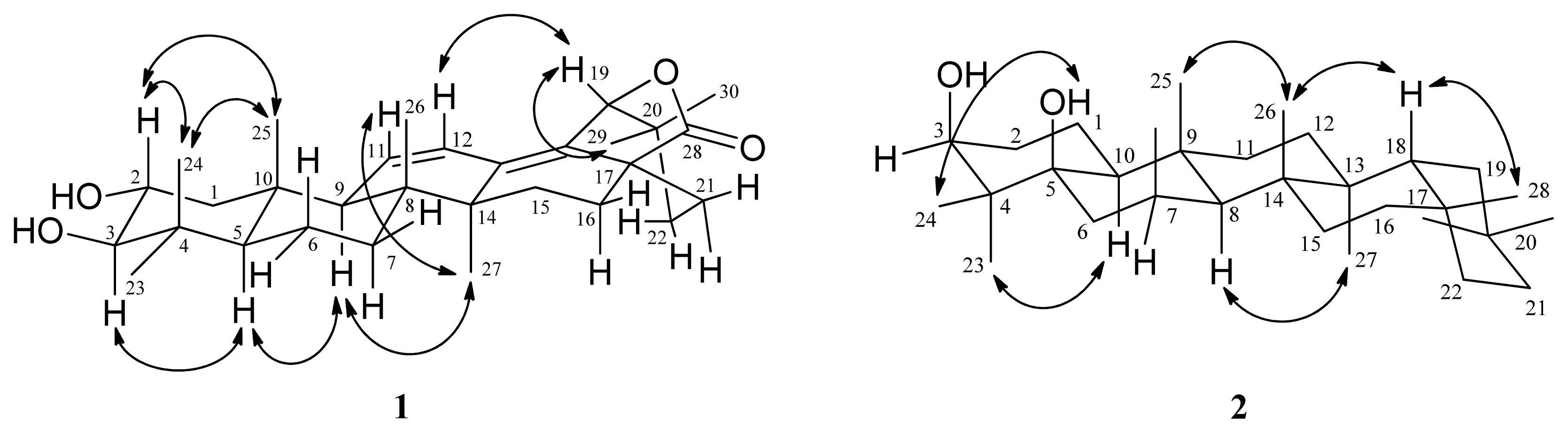
| position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 41.6 (CH2) | 0.97, m; 2.24, dd (12.4, 4.4) | 19.1 (CH2) | 1.52, m; 1.56, m |
| 2 | 68.8 (CH) | 3.77, ddd (9.6, 4.4, 2.0) | 30.6 (CH2) | 1.39, m; 1.78, m |
| 3 | 83.9 (CH) | 3.03, d ( 9.6) | 73.5 (CH) | 3.76, dd (11.2, 4.0) |
| 4 | 39.2 (C) | – | 43.9 (C) | – |
| 5 | 54.9 (CH) | 0.91, m | 77.4 (C) | – |
| 6 | 18.1 (CH2) | 1.44, m | 34.4 (CH2) | 1.55, m; 1.77, m |
| 7 | 33.0 (CH2) | 1.39, m; 1.46, m | 17.2 (CH2) | 1.45, m; 1.50, m |
| 8 | 41.0 (C) | – | 52.5 (CH) | 1.24, m |
| 9 | 52.8 (CH) | 2.14, br s | 36.8 (C) | – |
| 10 | 37.9 (C) | 2.40, m | 50.9 (CH) | 1.31, m |
| 11 | 129.1 (CH) | 5.77, dd (10.2, 2.0) | 34.9 (CH2) | 1.18, m; 1.54, m |
| 12 | 123.3 (CH) | 6.15, dd (10.2, 2.8 ) | 30.3 (CH2) | 1.31, m; 1.39, m |
| 13 | 134.7 (C) | – | 38.2 (C) | – |
| 14 | 40.6 (C) | – | 39.5 (C) | – |
| 15 | 25.5 (CH2) | 1.27, m; 1.39, m | 32.5 (CH2) | 1.28, m; 1.51, m |
| 16 | 24.2 (CH2) | 2.33, ddd (14.4, 5.2, 2.6) | 39.2 (CH2) | 0.92, m; 1.48, m |
| 17 | 44.0 (C) | – | 29.8 (C) | – |
| 18 | 132.9 (C) | – | 42.7 (CH) | 1.54. m |
| 19 | 85.0 (CH) | 4.72, s | 32.8 (CH2) | 1.28, m; 1.51, m |
| 20 | 35.7 (C) | – | 28.2 (C) | – |
| 21 | 32.6 (CH2) | 1.45, m; 1.62, m | 35.3 (CH2) | 1.18, m; 1.38, m |
| 22 | 34.5 (CH2) | 1.63, m; 1.83, m | 35.9 (CH2) | 1.37, m; 1.56, m |
| 23 | 28.3 (CH3) | 1.04, s | 19.2 (CH3) | 0.97, s |
| 24 | 16.3 (CH3) | 0.82, s | 16.5 (CH3) | 0.89, s |
| 25 | 19.1 (CH3) | 1.02, s | 17.1 (CH3) | 0.95, s |
| 26 | 16.9 (CH3) | 0.76, s | 20.4 (CH3) | 1.01, s |
| 27 | 19.4 (CH3) | 1.01, s | 18.7 (CH3) | 1.01, s |
| 28 | 178.1 (C) | – | 32.1 (CH3) | 1.17, s |
| 29 | 27.8 (CH3) | 1.10, s | 35.0 (CH3) | 0.94, s |
| 30 | 23.3 (CH3) | 0.95, s | 31.8 (CH3) | 0.99, s |
| OH-5 | – | – | – | 1.10, br s check |
| Compounds | IC50 (μM)a |
|---|---|
| 2α,3β-dihydroxyolean-11,13(18)-dien-19β,28-olide (1) | 98.37 ± 6.84 |
| 3β,5β-dihydroxyglutinol (2) | 56.08 ± 0.57 |
| glutinol (3) | >100 |
| 5(6)-gluten-3α-ol (4) | >100 |
| uvaol (5) | >100 |
| 20β,28-epoxy-28α-methoxytaraxasteran-3β-ol (6) | >100 |
| tormentic acid (7) | >100 |
| camaldulenic acid (8) | 89.42 ± 4.26 |
| 2α,3β-dihydroxyolean-13(18)-en-28-oic acid (9) | 16.50 ± 0.56 |
| Ibuprofenb | 27.53 ± 3.58 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, C.-H.; Chang, H.-S.; Liao, H.-R.; Chen, I.-S.; Tsai, I.-L. Triterpenoids from the Roots of Rhaphiolepis indica var. tashiroi and Their Anti-Inflammatory Activity. Int. J. Mol. Sci. 2013, 14, 8890-8898. https://doi.org/10.3390/ijms14058890
Lin C-H, Chang H-S, Liao H-R, Chen I-S, Tsai I-L. Triterpenoids from the Roots of Rhaphiolepis indica var. tashiroi and Their Anti-Inflammatory Activity. International Journal of Molecular Sciences. 2013; 14(5):8890-8898. https://doi.org/10.3390/ijms14058890
Chicago/Turabian StyleLin, Chu-Hung, Hsun-Shuo Chang, Hsiang-Ruei Liao, Ih-Sheng Chen, and Ian-Lih Tsai. 2013. "Triterpenoids from the Roots of Rhaphiolepis indica var. tashiroi and Their Anti-Inflammatory Activity" International Journal of Molecular Sciences 14, no. 5: 8890-8898. https://doi.org/10.3390/ijms14058890





