Biodegradable Polycaprolactone-Titania Nanocomposites: Preparation, Characterization and Antimicrobial Properties
Abstract
:1. Introduction
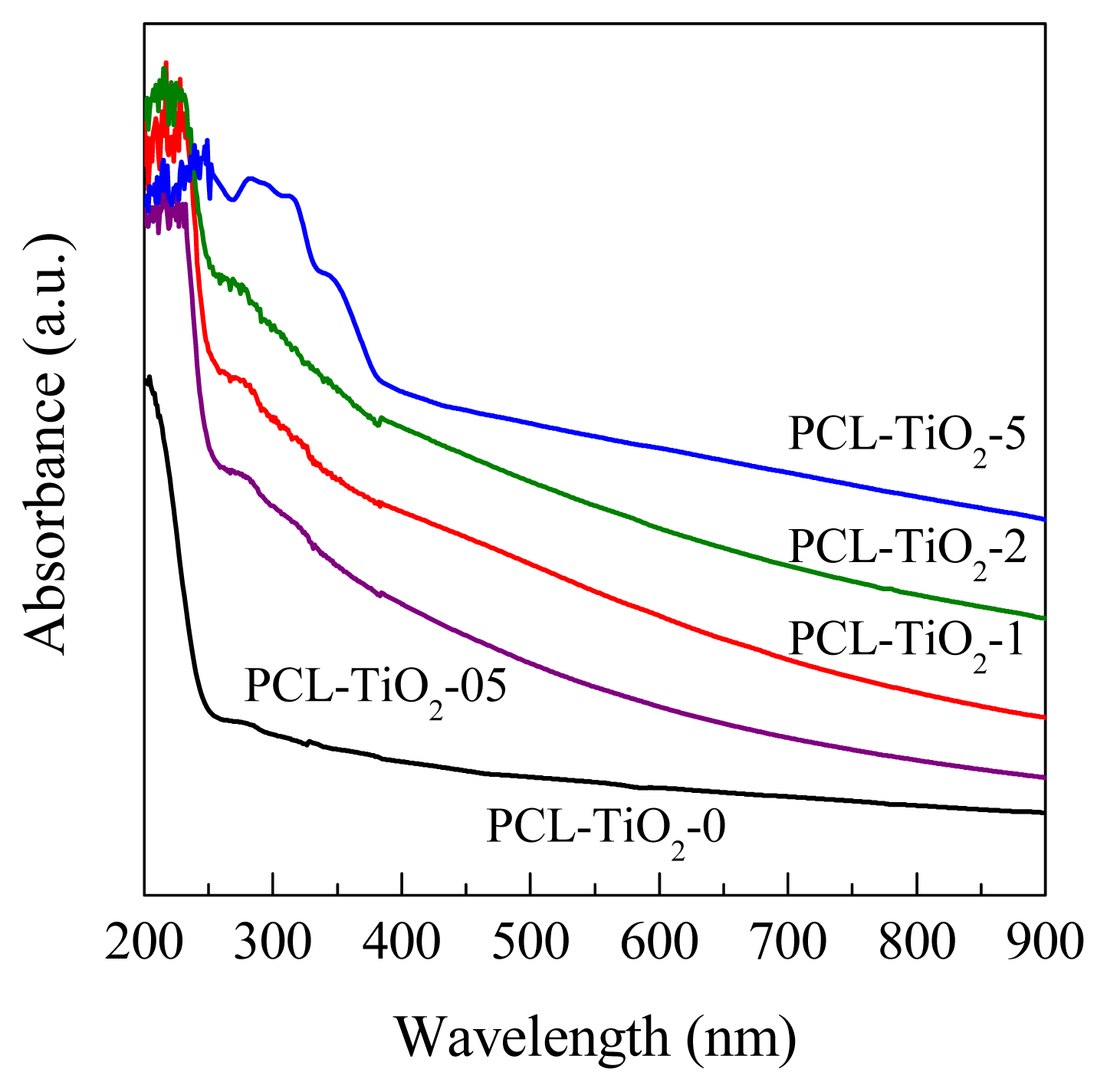
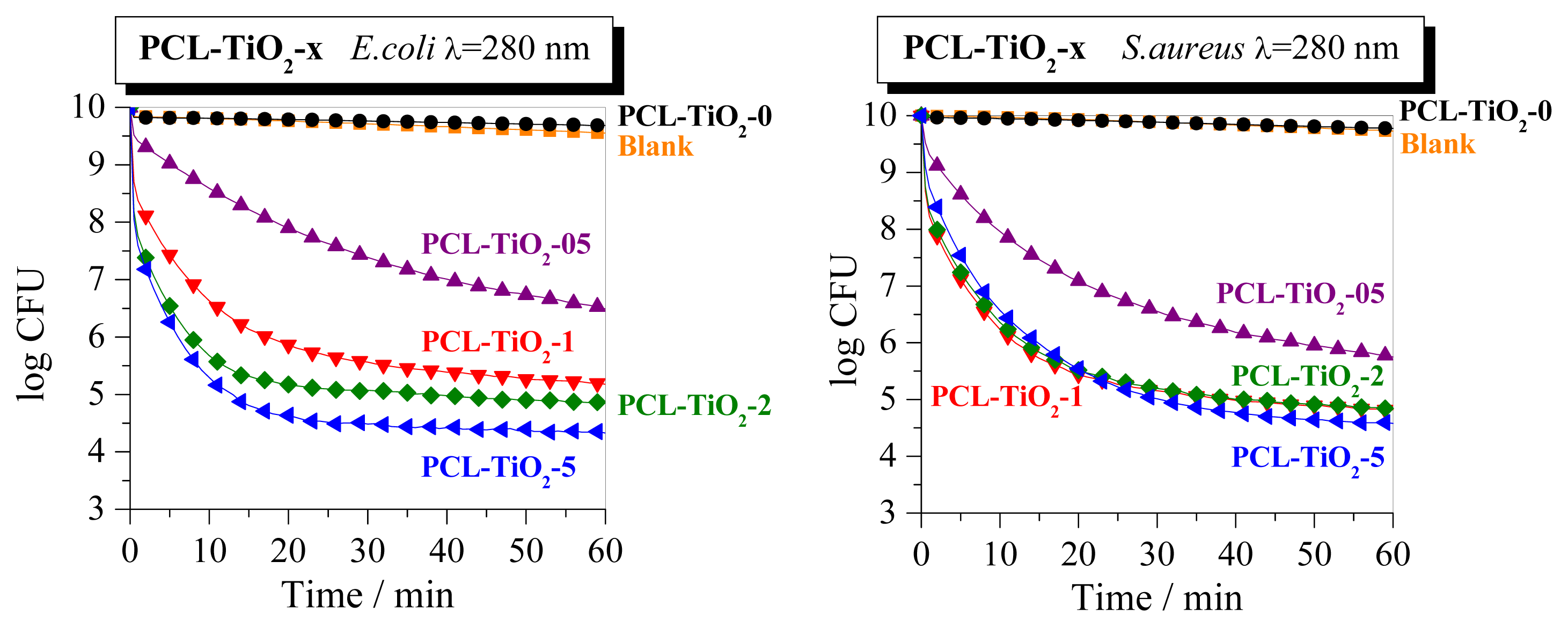
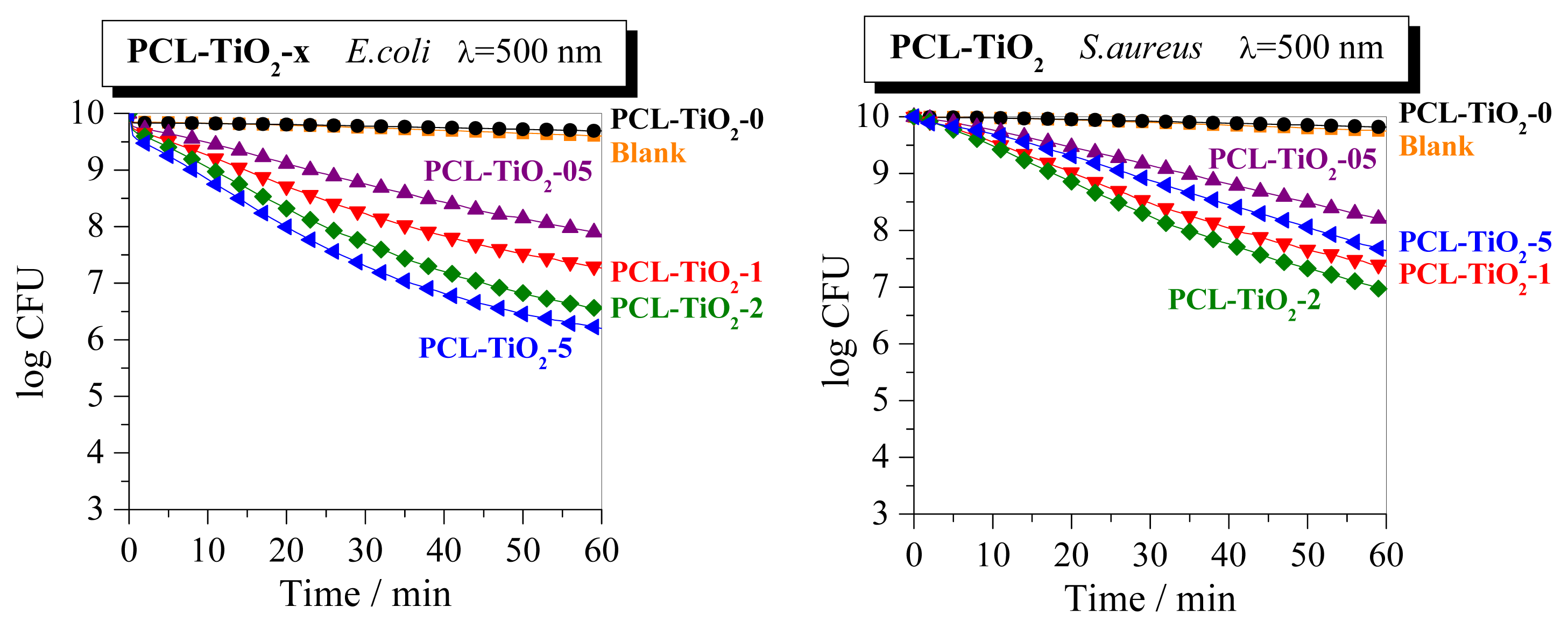
2. Results and Discussion
3. Experimental Section
4. Conclusions
Acknowledgments
Conflict of Interest
References
- John, J.; Mani, R.; Battacharya, M. Evaluation compatibility and properties of biodegradable polyester blends. J. Polym. Sci. Polym. Chem 2002, 40, 2003–2014. [Google Scholar]
- Cao, A.; Okamura, T.; Ishiguro, C.; Nakayama, K.; Inoue, Y.; Masuda, T. Studies on synthesis and physical characteristics of biodegradable aliphatic poly (butylene succinate-co-ɛ-caprolactone). Polymer 2002, 43, 671–679. [Google Scholar]
- Sarasam, A.R.; Samli, A.I.; Hess, L.; Ihnat, M.A.; Madihally, S.V. Blending chitosan with polycaprolactone: Porous scaffolds and toxicity. Macromol. Biosci 2007, 7, 1160–1167. [Google Scholar]
- Nohynek, G.J.; Lademann, J.; Ribaud, C.; Roberts, M.S. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit. Rev. Toxicol 2007, 37, 251–277. [Google Scholar]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol 2002, 3, 113–126. [Google Scholar]
- Jacoby, W.A.; Maness, P.C.; Wolfrum, E.J.; Blake, D.M.; Fennell, J.A. Mineralization of bacterial cell mass on a photocatalytic surface in air. Environ. Sci. Technol 1998, 32, 2650–2653. [Google Scholar]
- Brook, L.A.; Evans, P.; Foster, H.A.; Pemble, M.E.; Steele, A.; Sheel, D.W.; Yates, H.M. Highly bioactive silver and silver/titania composite films grown by chemical vapour deposition. J. Photochem. Photobiol. A 2007, 187, 53–63. [Google Scholar]
- Dietbol, U. The surface science of TiO2. Surf. Sci. Rep 2003, 48, 53–229. [Google Scholar]
- Fernández-García, M.; Martínez-Arias, A.; Hanson, J.C.; Rodríguez, J.A. Nanostructured oxides in chemistry: Characterization and properties. Chem. Rev 2004, 104, 4063–4104. [Google Scholar]
- Kubacka, A.; Serrano, C.; Ferrer, M.; Lünsdorf, H.; Bielecki, P.; Cerrada, M.L.; Fernández-García, M.; Fernández-García, M. High performance dual action polymer TiO2 nanocomposite films via melting processing. Nano Lett 2007, 7, 2529–2534. [Google Scholar]
- Kubacka, A.; Cerrada, M.L.; Serrano, C.; Fernández-García, M.; Ferrer, M.; Fernández-García, M. Light-driven novel properties of TiO2-modified Polypropylene-based nanocomposite films. J. Nanosci. Nanotech 2008, 8, 3241–3246. [Google Scholar]
- Cerrada, M.L.; Serrano, C.; Sánchez-Chaves, M.; Fernández-García, M.; Fernández-Martín, F.; de Andrés, A.; Jiménez Riobóo, R.J.; Kubacka, A.; Ferrer, M.; Fernández-García, M. Self-sterilized EVOH-TiO2 nanocomposites: Effect of TiO2 content on biocidal properties. Adv. Funct. Mater 2008, 18, 1949–1960. [Google Scholar]
- Cerrada, M.L.; Serrano, C.; Sánchez-Chaves, M.; Fernández-García, M.; de Andrés, M.A.; Jiménez Riobóo, R.J.; Fernández-Martín, F.; Kubacka, A.; Ferrer, M.; Fernández-García, M. Biocidal capability optimization in organic inorganic nanocomposites based on titania. Environ. Sci. Techn 2009, 43, 1630–1634. [Google Scholar]
- Gómez-Romero, P.; Sánchez, C. Functional Hybrid Materials; Wiley-Interscience: New York, NY, USA, 2004. [Google Scholar]
- Sanchez, C.; Julián, B.; Belleiville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem 2005, 15, 3559–3592. [Google Scholar]
- Kubacka, A.; Ferrer, M.; Cerrada, M.L.; Serrano, C.; Sánchez-Chaves, M.; Fernández-García, M.; de Andrés, A.; Jiménez Riobóo, R.J.; Fernández-Martín, F.; Fernández-García, M. Boosting TiO2-anatase antimicrobial activity: Polymer-oxide thin films. Appl. Catal. B Environ 2009, 89, 441–447. [Google Scholar]
- Kubacka, A.; Ferrer, M.; Fernández-García, M.; Serrano, C.; Cerrada, M.L.; Fernández-García, M. Tailoring polymer-TiO2 properties by presence of metal (Ag, Cu, Zn) species: Optimization of antimicrobial properties. Appl. Catal. B Environ 2011, 104, 346–352. [Google Scholar]
- Hu, H.; Dorset, D.L. Crystal structure of poly(ɛ-capro1actone). Macromolecules 1990, 23, 4604–4607. [Google Scholar]
- Bahloul, W.; Bounor-Legaré, V.; David, L.; Cassagnau, P. Morphology and viscoelasticity of PP/TiO2 nanocomposites prepared by in situ sol–gel method. J. Polym. Sci. Part B Polym. Phys 2010, 48, 1213–1222. [Google Scholar]
- Baltá-Calleja, F.J.; Vonk, C.G. X-ray Scattering of Synthetic Polymers; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Ryan, A.J.; Stanford, J.L.; Bras, W.; Nye, T.M.W. A synchrotron X-ray study of melting and recrystallization in isotactic polypropylene. Polymer 1997, 38, 759–768. [Google Scholar]
- Crist, B. Small-angle X-ray scattering and polymer melting: A model study. J. Polym. Sci. Part B Polym. Phys 2001, 39, 2454–2460. [Google Scholar]
- Lee, Y.; Porter, R.S.; Lin, J.S. On the double-melting behaviour of poly (ether ether ketone). Macromolecules 1989, 22, 1756–1760. [Google Scholar]
- Cerrada, M.L.; Benavente, R.; Pérez, E. Crystalline structure and viscoelastic behavior in composites of a metallocenic ethylene-1-octene copolymer and glass fiber. Macromol. Chem. Phys 2002, 203, 718–726. [Google Scholar]
- Arranz-Andrés, J.; Peña, B.; Benavente, R.; Pérez, E.; Cerrada, M.L. Influence of isotacticity and molecular weight on the properties of metallocenic isotactic polypropylene. Eur. Polym. J 2007, 43, 2357–2370. [Google Scholar]
- López-Majada, J.M.; Palza, H.; Guevara, J.L.; Quijada, R.; Martínez, M.C.; Benavente, R.; Pereña, J.M.; Pérez, E.; Cerrada, M.L. Metallocenic copolymers of propene and 1 hexene: Influence of comonomer content and thermal history on the structure and mechanical properties. J. Polym. Sci. Part B Polym. Phys 2006, 44, 1253–1267. [Google Scholar]
- Bassett, D.C.; Olley, R.H.; Al Raheil, I.A.M. On isolated lamellae of melt-crystallized polyethylene. Polymer 1988, 29, 1745–1754. [Google Scholar]
- Arranz-Andrés, J.; Benavente, R.; Ribeiro, M.R.; Pérez, E.; Cerrada, M.L. Evolution of a metallocenic sPP with time: Changes in crystalline content and enthalpic relaxation. Macromol. Chem. Phys 2006, 207, 1564–1574. [Google Scholar]
- Arranz-Andrés, J.; Benavente, R.; Pérez, E.; Cerrada, M.L. Structure and mechanical behavior of the mesomorphic form in a propylene b EPR copolymer and its comparison with other thermal treatments. Polym. J 2003, 35, 766–777. [Google Scholar]
- Krache, R.; Benavente, R.; López-Majada, J.M.; Pereña, J.M.; Cerrada, M.L.; Pérez, E. Competition between alpha, beta and gamma polymorphs in a beta-nucleated metallocene isotactic polypropylene. Macromolecules 2007, 40, 6871–6878. [Google Scholar]
- Cerrada, M.L.; Polo-Corpa, M.J.; Benavente, R.; Pérez, E.; Velilla, T.; Quijada, R. Formation of the new trigonal polymorph in iPP-1-Hexene copolymers. Competition with the mesomorphic phase. Macromolecules 2009, 42, 702–708. [Google Scholar]
- Pérez, E.; Gómez-Elvira, J.M.; Benavente, R.; Cerrada, M.L. Tailoring the formation rate of the mesophase in random propylene-co-1-pentene copolymers. Macromolecules 2012, 45, 6481–6490. [Google Scholar]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ɛ-caprolactone. comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J 1972, 8, 449–463. [Google Scholar]
- Nakagawa, S.; Kadena, K.; Ishizone, T.; Nojima, S.; Shimizu, T.; Yamaguchi, K.; Nakahama, S. Crystallization behavior and crystal orientation of poly(ɛ-caprolactone) homopolymers confined in nanocylinders: Effects of nanocylinder dimension. Macromolecules 2012, 45, 1892–1900. [Google Scholar]
- Matyi, R.J.; Schwartz, L.H.; Butt, J.B. Particle size, particle size distribution, and related measurements of supported metal catalysts. Catal. Rev. Sci. Eng 1987, 29, 41–99. [Google Scholar]
- Arranz-Andrés, J.; Pulido-González, N.; Marín, P.; Aragón, A.; Cerrada, M.L. Electromagnetic shielding features in lightweight PVDF aluminum based nanocomposites. Prog. Electromagn. Res. B 2013, 48, 175–196. [Google Scholar]
- Cerrada, M.L.; Pérez, E.; Lourenço, J.P.; Campos, J.M.; Ribeiro, M.R. Hybrid HDPE/MCM-41 nanocomposites: Crystalline structure and viscoelastic behaviour. Micropor. Mesopor. Mat 2010, 130, 215–223. [Google Scholar]
- Arranz-Andrés, J.; Pérez, E.; Cerrada, M.L. Hybrids based on poly(vinylidene fluoride) and Cu nanoparticles: Characterization and EMI shielding. Eur. Polym. J 2012, 48, 1160–1168. [Google Scholar]
- Serrano, C. Oxide-polymer antimicrobial nanocomposites: Chemical-physics characterization and biocidal response. Ph.D. Thesis, Univesidad Complutense de Madrid, Madrid, Spain, 2011. [Google Scholar]
- Serrano, C.; Cerrada, M.L.; Fernández-García, M.; Ressia, J.; Vallés, E.M. Rheological and structural details of biocidal iPP-TiO2 nanocomposites. Eur. Polym. J 2012, 48, 586–596. [Google Scholar]
- Kubacka, A.; Ferrer, M.; Fernández-García, M. Kinetics of photocatalytic disinfection in TiO2-containing polymer thin films. Appl. Catal. B Environ. 2012, 121–122, 230–238. [Google Scholar]
- Blackburn, C. Food Spoilage Microorganisms; Woodhead Publishing Ltd: Cambridge, UK, 2006. [Google Scholar]
- Gunndermann, K.O.; Ruden, H.; Sunntag, H.G. Lehrbuch der Hygiene; Ficher: Stuttgart, Germany & New York, NY, USA, 1991. [Google Scholar]
- Huang, Z.; Maness, P.C.; Blake, D.M.; Wolfrum, E.J.; Smolinski, S.L.; Jacoby, W.A. Bactericidal mode of titanium dioxide photocatalysis. J. Photochem. Photobiol. A 2000, 130, 163–170. [Google Scholar]
- Ibañez, J.A.; Litter, M.I.; Pizarro, R.A. Photocatalytic bactericidal effect of TiO2 on enterobacter cloacae: Comparative study with other Gram (−) bacteria. J. Photochem. Photobiol. A 2003, 157, 81–85. [Google Scholar]
- Robertson, J.M.C.; Robertson, P.K.; Lawton, L.A. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic micro-organisms. J. Photochem. Photobiol. A 2005, 175, 51–56. [Google Scholar]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res 2004, 38, 1069–1077. [Google Scholar]
- Joo, J.; Kwon, S.G.; Yu, T.; Cho, M.; Lee, J.; Yoon, J.; Hyeon, T. Large-scale synthesis of TiO2 nanorods via nonhydrolytic sol–gel ester elimination reaction and their application to photocatalytic inactivation of E. coli. J. Phys. Chem. B 2005, 109, 15297–15302. [Google Scholar]
- Lonnen, J.; Kilvington, S.; Al-Touati, F.; McGuigan, K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res 2005, 39, 877–883. [Google Scholar]
- You, K.Y.; Byeon, J.H.; Park, J.H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ 2007, 373, 572–575. [Google Scholar]
- Ho, C.; Tobis, J.; Sprich, C.; Thomann, R.; Tiller, J.C. Nanoseparated polymeric networks with multiple antimicrobial properties. Adv. Mater 2004, 16, 957–961. [Google Scholar]
- Arnol, J.W.; Boothe, D.H.; Mitatell, B.W. Use of negative air ionization for reducing bacterial pathogens and spores on stainless steel surfaces. J. Appl. Poult. Res 2004, 13, 200–206. [Google Scholar]
- Seven, O.; Dinder, B.; Aydemir, S.; Metin, D.; Ozinel, M.A.; Icli, S. Solar photocatalytic disinfection of a group of bacteria and fungi aqueous suspensions with TiO2, ZnO and Sahara desert dust. J. Photochem. Photobiol. A 2004, 165, 103–107. [Google Scholar]
- Kuhn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W.; Sonnatg, H.-G.; Erdinger, L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar]
- Wang, Z.; Li, G.; Peng, H.; Zhang, Z.; Wang, X. Study on novel antibacterial high impact polystyrene/TiO2 nanocomposites. J. Mater. Sci 2005, 40, 6433–6438. [Google Scholar]
- Page, K.; Palgrave, R.G.; Parkin, P.; Wilson, M.; Sarin, S.L.P.; Chadwick, A.V. Titania and silver-titania composite films on glass-potent antimicrobial coatings. J. Mater. Sci 2007, 17, 95–104. [Google Scholar]
- Vohra, A.; Goswami, D.Y.; Deshpande, D.A.; Block, S.S. Enhanced photocatalytic disinfection of indoor air. Appl. Catal. B 2006, 65, 57–65. [Google Scholar]
- Cowan, M.M.; Abshire, K.Z.; Houk, S.L.; Evans, S.M. Antimicrobial efficacy of a silver-zeolite matrix coating on stainless steel. J. Ind. Microbiol. Biotechnol 2003, 30, 102–106. [Google Scholar]
- Oyama, A.; Yokoyama, Y.; Uchida, M.; Ito, A. The formation of an antibacterial agent–apatite composite coating on a polymer surface using a metastable calcium phosphate solution. Biomaterials 2006, 27, 3295–3303. [Google Scholar]
- Rincón, A.G.; Pulgarin, C. Photocatalytical inactivation of E. coli: Effect of (continuous-intermittent) light intensity and of (suspended-fixed) TiO2 concentration. Appl. Catal. B 2003, 44, 263–284. [Google Scholar]
- Rincón, A.G.; Pulgarin, C. Bactericidal action of illuminated TiO2 on pure Escherichia coli and natural bacterial consortia: Post-irradiation events in the dark and assessment of the effective disinfection time. Appl. Catal. B 2004, 49, 99–112. [Google Scholar]
- Bahloul, W.; Mélisa, F.; Bounor-Legaréa, V.; Cassagnau, P. Structural characterisation and antibacterial activity of PP/TiO2 nanocomposites prepared by an in situ sol–gel method. Mat. Chem. Phys 2012, 134, 399–406. [Google Scholar]
- Kubacka, A.; Cerrada, M.L.; Serrano, C.; Fernández-García, M.; Ferrer, M.; Fernández-García, M. Plasmonic nanoparticle/polymer nanocomposites with enhanced photocatalytic antimicrobial properties. J. Phys. Chem. C 2009, 113, 9182–9190. [Google Scholar]
- Fernández-García, M.; Wang, X.; Belver, C.; Hanson, J.C.; Rodriguez, J.A. Anatase-TiO2 nanomaterials: Morphological/size dependence of the crystallization and phase behavior phenomena. J. Phys. Chem. C 2007, 111, 674–682. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Ferrer, M.; Soliveri, J.; Plou, F.J.; López-Cortés, N.; Reyes-Duarte, D.; Christensen, M.; Copa-Patiño, J.L.; Ballesteros, A. Synthesis of sugar esters in solvent mixtures by lipases from thermomyces lanuginosus and Candida antarctica B, and their antimicrobial properties. Enzyme Microb. Technol 2005, 36, 391–398. [Google Scholar]
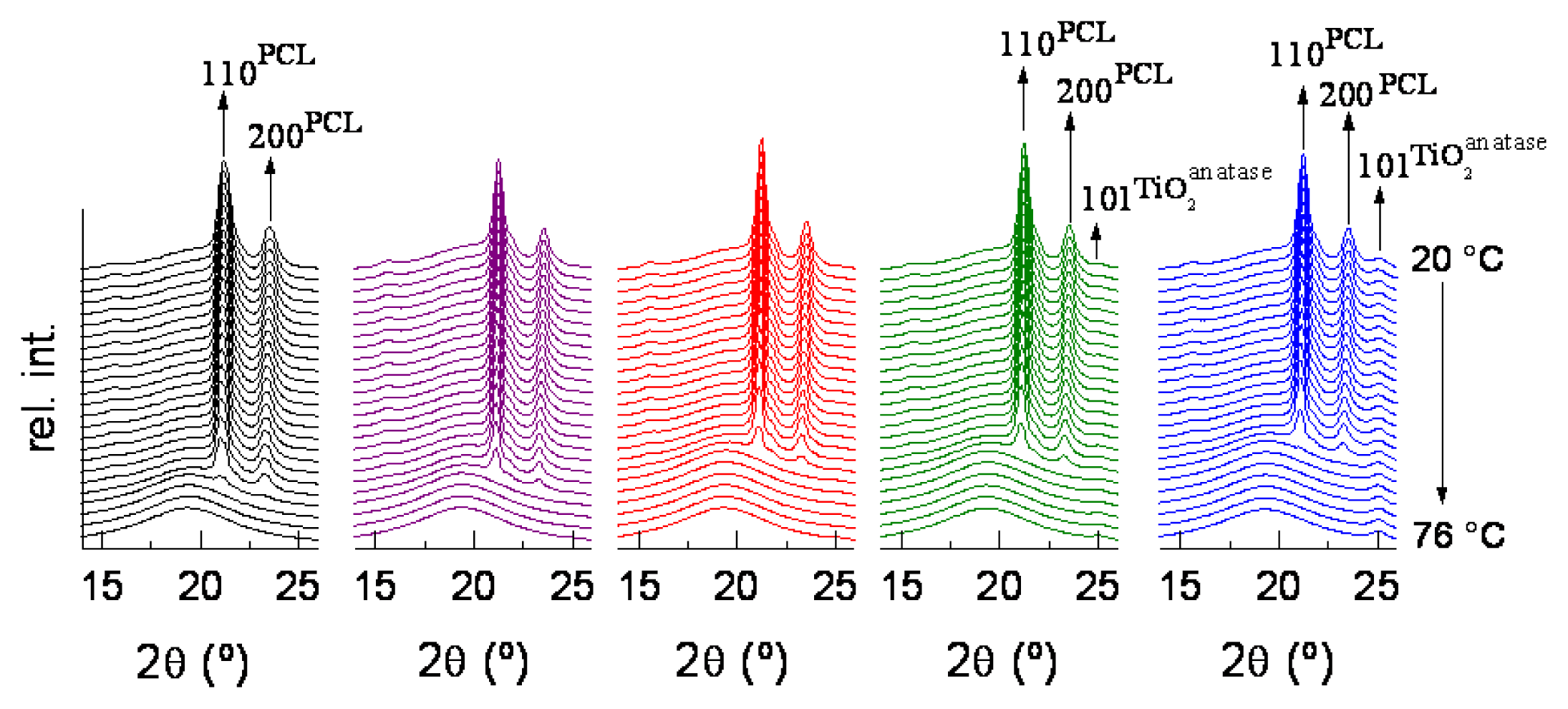
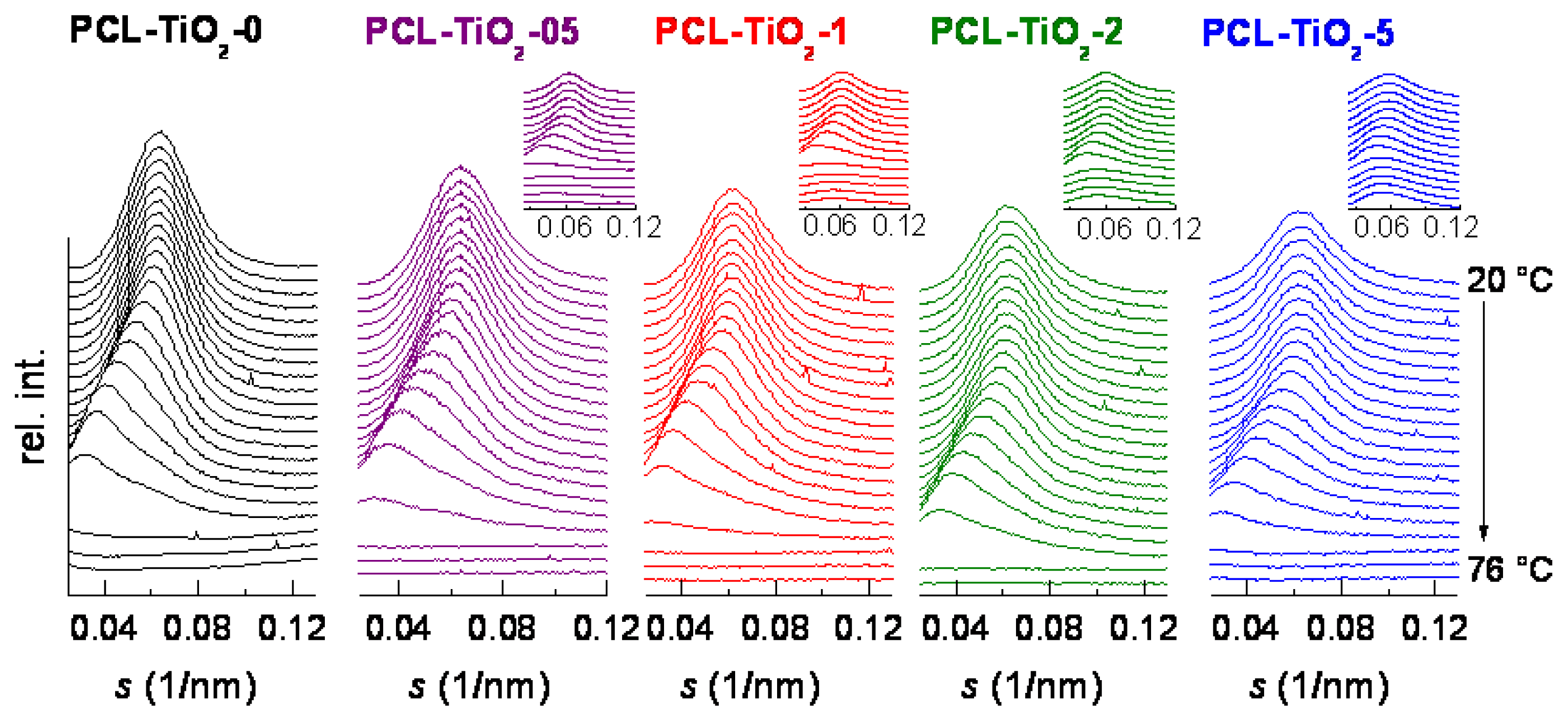

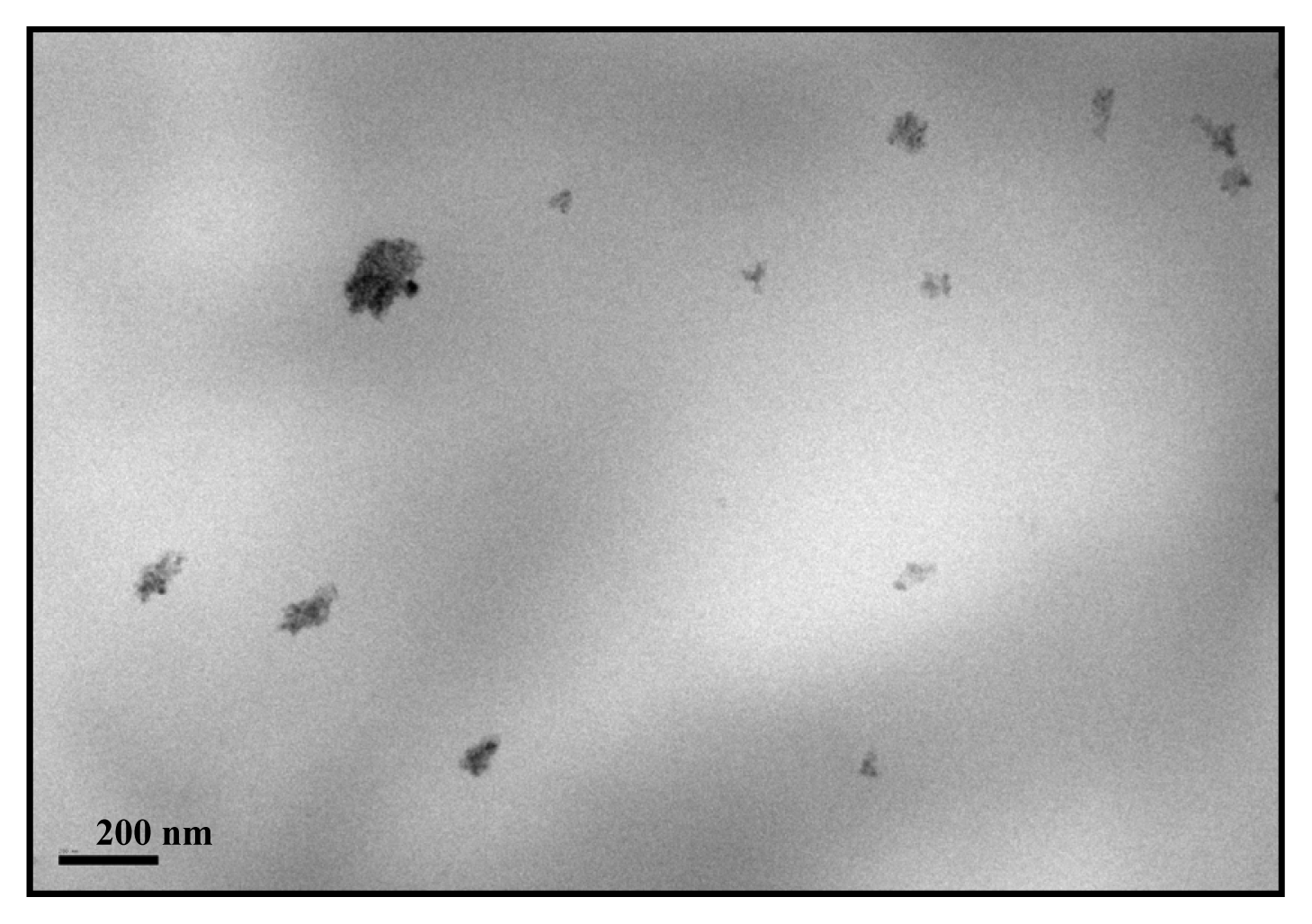
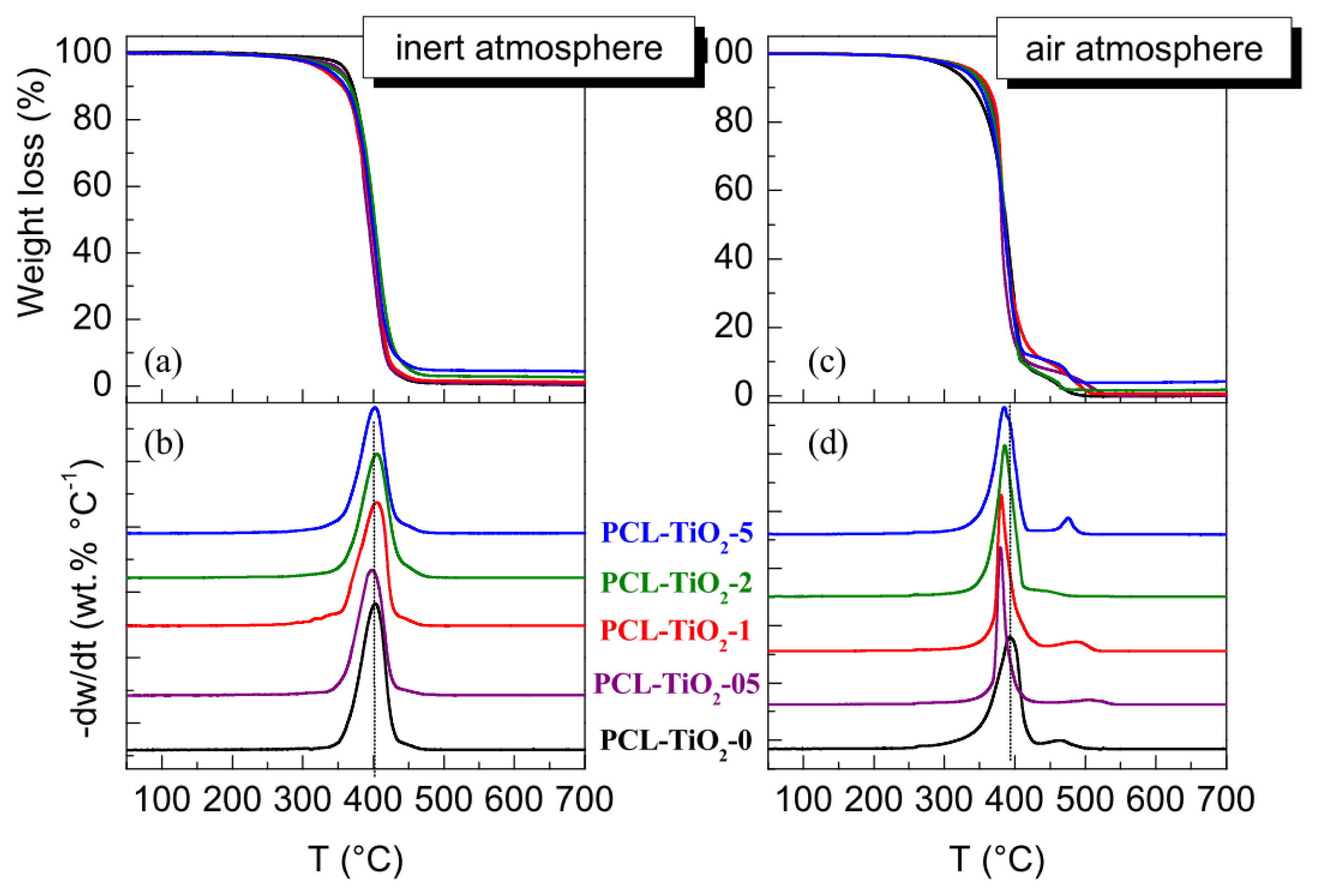
| Specimen | TiO2 wt.% | fcNORMWAXS | dHALO (nm) | LSAXS (nm) | lc (nm) |
|---|---|---|---|---|---|
| PCL-TiO2-0 | 0.0 | 0.53 | 0.448 | 15.8 | 8.4 |
| PCL-TiO2-05 | 0.5 | 0.54 | 0.448 | 15.8 | 8.5 |
| PCL-TiO2-1 | 1.0 | 0.54 | 0.449 | 16.1 | 8.7 |
| PCL-TiO2-2 | 2.0 | 0.53 | 0.449 | 16.1 | 8.5 |
| PCL-TiO2-5 | 5.0 | 0.53 | 0.450 | 16.0 | 8.5 |
| Specimen | Tg (°C) | fcNORMF1 | TmF1 (°C) | fcNORMC | Tc (°C) | fcNORMF2 | TmF2 (°C) |
|---|---|---|---|---|---|---|---|
| PCL-TiO2-0 | −60.0 | 0.68 | 60.0 | 0.47 | 32.5 | 0.59 | 55.0 |
| PCL-TiO2-05 | −60.5 | 0.66 | 59.5 | 0.47 | 36.0 | 0.59 | 55.5 |
| PCL-TiO2-1 | −60.5 | 0.66 | 59.5 | 0.46 | 36.0 | 0.58 | 56.0 |
| PCL-TiO2-2 | −60.0 | 0.66 | 59.5 | 0.47 | 34.5 | 0.59 | 55.5 |
| PCL-TiO2-5 | −60.0 | 0.65 | 60.0 | 0.46 | 34.0 | 0.58 | 55.5 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Muñoz-Bonilla, A.; Cerrada, M.L.; Fernández-García, M.; Kubacka, A.; Ferrer, M.; Fernández-García, M. Biodegradable Polycaprolactone-Titania Nanocomposites: Preparation, Characterization and Antimicrobial Properties. Int. J. Mol. Sci. 2013, 14, 9249-9266. https://doi.org/10.3390/ijms14059249
Muñoz-Bonilla A, Cerrada ML, Fernández-García M, Kubacka A, Ferrer M, Fernández-García M. Biodegradable Polycaprolactone-Titania Nanocomposites: Preparation, Characterization and Antimicrobial Properties. International Journal of Molecular Sciences. 2013; 14(5):9249-9266. https://doi.org/10.3390/ijms14059249
Chicago/Turabian StyleMuñoz-Bonilla, Alexandra, María L. Cerrada, Marta Fernández-García, Anna Kubacka, Manuel Ferrer, and Marcos Fernández-García. 2013. "Biodegradable Polycaprolactone-Titania Nanocomposites: Preparation, Characterization and Antimicrobial Properties" International Journal of Molecular Sciences 14, no. 5: 9249-9266. https://doi.org/10.3390/ijms14059249





