Thrombospondin-1 in Urological Cancer: Pathological Role, Clinical Significance, and Therapeutic Prospects
Abstract
:1. Introduction
2. Structure and Function of Thrombospondin
2.1. TSP-1
2.2. TSP-1 in Malignancies
3. TSP-1 in Prostate Cancer
3.1. TSP-1 Expression
3.2. Correlation with Clinicopathological Features
3.3. TSP-1 and Androgen Therapy
4. TSP-1 in Renal Cell Carcinoma
4.1. Pathological Significance of TSP-1
4.2. Correlation between TSP-1 Expression and Malignant Aggressiveness
5. TSP-1 and Urothelial Cancer
5.1. Pathological Significance
5.2. Correlation between TSP-1 Expression and Prognosis
6. Molecular Regulation by TSP-1
7. Fragments Derived from TSP-1
8. Treatment with TSP-1 Mimetic Drugs
9. Conclusions
Acknowledgements
Conflict of Interest
References
- Bouck, N.; Stellmach, V.; Hsu, S.C. How tumors become angiogenic. Adv. Cancer Res 1996, 69, 135–174. [Google Scholar]
- Battegay, E.J. Angiogenesis: Mechanistic insights, nanovascular diseases, and therapeutic prospects. J. Mol. Med 1995, 73, 333–346. [Google Scholar]
- Aragon-Ching, J.B.; Madan, R.A.; Dahut, W.T. Angiogenesis inhibition in prostate cancer: Current uses and future promises. J. Oncol. 2010. [Google Scholar] [CrossRef]
- Cohen, R.B.; Oudard, S. Antiangiogenic therapy for advanced renal cell carcinoma: Management of treatment-related toxities. Invest. New Drugs 2012, 30, 2066–2079. [Google Scholar]
- Vailhé, B.; Feige, J.J. Thrombospondins as anti-angiogenic therapeutic agents. Curr. Pharm. Des 2003, 9, 583–588. [Google Scholar]
- Pili, R.; Häggman, M.; Stadler, W.M.; Gingrich, J.; Assikis, V.; Björk, A.; Nordle, Ö.; Fosrsberg, G.; Carducci, M.A.; Armstrong, A.J. Phase II randomized double blind placebo-controlled study to determine the efficacy of tasquinimod in asymptomatic patients with metastatic castrate-resistant prostate cancer. J. Clin. Oncol 2011, 29, 4022–4028. [Google Scholar]
- Adams, J.C.; lawler, J. The thrombospondins. Int. J. Biochem. Cell Biol 2004, 36, 961–968. [Google Scholar]
- Adams, J.C. Thronbospondins: Multifuctional regulators of cell interactions. Annu. Rev. Cell Div. Biol 2001, 17, 25–51. [Google Scholar]
- Lawler, J.; Slayter, H.S.; Coligan, J.E. Isolated and characterisation of a high molecular weight glycoprotein from human blood platelet. J. Biol. Chem 1978, 253, 8609–8616. [Google Scholar]
- Isenberg, J.S.; Romeo, M.J.; Yu, C.; Yu, C.K.; Nghiem, K.; Monsale, J.; Rick, M.E.; Wink, D.A.; Fraizier, W.A.; Roberts, D.D. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 2008, 111, 613–623. [Google Scholar]
- Jaffe, E.A.; Ruggiero, J.T.; Falcone, D.J. Monocytes and macrophages synthesize and secrete thrombospondin. Blood 1985, 65, 78–84. [Google Scholar]
- Majack, R.A.; Goodman, L.V.; Dixit, V.M. Cell surface thrombospondin is functionally essential for vascular smooth muscle cells proliferation. J. Cell Biol 1988, 106, 415–422. [Google Scholar]
- Bornstein, P. Thoronbospondins as matricelular modulators of cell function. J. Clin. Invest 2001, 107, 929–934. [Google Scholar]
- Reed, M.J.; Puolakkainen, P.; Lane, T.F.; Dickerson, D.; Bornstein, P.; Sage, E.H. Different expression of SPARC and thrombospondin 1 in wound repair: Immunolocalization and in situ hybridization. J. Histochem. Cytochem 1993, 41, 1467–1477. [Google Scholar]
- Bauer, E.M.; Qin, Y.; Miller, T.W.; Bandle, R.W.; Csanyi, G.; Pagano, P.J.; Bauer, P.M.; Schnermann, J.; Roberts, D.D.; Isenberg, J.S. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc. Res 2010, 88, 471–481. [Google Scholar]
- Lopez-Dee, Z.; Pidcock, K.; Gutierrez, L.S. Thrombospondin-1: Multiple paths to inflammation. Mediators Inflamm. 2011. [Google Scholar] [CrossRef]
- Bornstein, P. Diversity of function is inherent in matricelluar proteins: An appraisal of thrombospondin 1. J. Cell Biol 1995, 130, 503–506. [Google Scholar]
- Hugo, C.; Daniel, C. Thrombospondin in renal disease. Exper. Nephrol 2009, 111, e61–e66. [Google Scholar]
- Qian, X.; Tuszynski, G.P. Expression of thrombospondin-1 in cancer: A role in tumor progression. Proc. Soc. Exp. Biol. Med 1996, 212, 199–207. [Google Scholar]
- Taraboletti, G.; Roberts, D.D.; Liotta, L.A.; Giavazzi, R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: A potential angiogenesis regulatory factor. J. Cell Biol 1990, 111, 765–772. [Google Scholar]
- Tolsma, S.S.; Stack, M.S.; Bouck, N. Lumen formation and other angiogenic activities of cultured capillary endothelial cells are inhibited by thorombospondin-1. Microvasc. Res 1997, 54, 13–26. [Google Scholar]
- Jimenez, B.; Volpert, O.V.; Crawford, S.E.; Febbraio, M.; Silverstein, R.L.; Bouck, N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat. Med 2000, 6, 41–48. [Google Scholar]
- Cambells, S.C.; Volpert, O.V.; Ivanovich, M.; Bouck, N.P. Molecular mediators of angiogenesis in bladder cancer. Cancer Res 1998, 58, 1298–1304. [Google Scholar]
- Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell Mol. Med 2002, 6, 1–12. [Google Scholar]
- Armstrong, L.C.; Bormstein, P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol 2003, 22, 63–71. [Google Scholar]
- Karavasilis, V.; malamou-Mitsi, V.; Briasoulis, E.; Tsanou, E.; Kitsou, E.; Pavlidis, N. Clinicopathological study of vascular endothelial growth factor, thrombospondin-1, and microvessel density assessed by CD34 in patients with stage III ovarian carcinoma. Int. J. Gynecol. Cancer 2006, 16, 241–246. [Google Scholar]
- Qian, X.; Wang, T.N.; Rothman, V.L.; Nicosia, R.F.; Tuszynski, G.P. Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp. Cell Res 1997, 235, 403–412. [Google Scholar]
- Dawson, D.M.; Pearce, S.F.; Zhong, R.; Silverstein, R.L.; Frazier, W.A.; Bouck, N.P. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 have anti-angiogenic activity. J. Cell Biol 1997, 138, 7070–7717. [Google Scholar]
- Magnetto, S.; Bruno-Bossio, G.; Voland, C.; Lecerf, J.; Lawler, J.; Delmas, P.; Silverstein, R.; Clezardin, P. CD36 mediates binding of soluble thrombospondin-1 but not cell adhesion and haptotaxis on immobilized thrombospondin-1. Cell Biochem. Funct 1998, 16, 211–221. [Google Scholar]
- Lawler, J. The functions of TSP-1 and TSP-2a. Curr. Opin. Cell Biol 2000, 12, 634–650. [Google Scholar]
- Isenberg, J.S.; Martin-Manso, G.; Maxhimer, J.B.; Roberts, D.D. Regulation of nitric oxide signaling by thrombospondin 1: Implications for anti-angiogenic therapies. Nat. Rev. Cancer 2009, 9, 182–194. [Google Scholar]
- Roberts, D.D. Regulation of tumor growth and metastasis by thrombospondin-1. FASEB. J 1996, 10, 1183–1191. [Google Scholar]
- Bertin, N.; Clezardin, P.; Kubiak, R.; Frappart, T. Thrombospondin-1 and -2 messenger RNA expression in normal, benign, and neoplastic human breast tissues: Correlation with prognostic factors, tumor angiogenesis, and fibroblastic desmoplasia. Cancer Res 1997, 57, 396–399. [Google Scholar]
- Yoshida, Y.; Oshika, Y.; Fukushima, Y.; Tokunaga, T.; Hatanaka, H.; Kijima, H.; Yamazaki, H.; Ueyama, Y.; Tamaoki, N.; Miura, S.; et al. Expression of angiostatic factors in colorectal cancer. Int. J. Oncol 1999, 15, 1221–1225. [Google Scholar]
- Oshiba, G.; Kijima, H.; Himeno, S.; Kenmochi, T.; Kise, Y.; Tanaka, H.; Nishi, T.; Chino, O.; Shimada, H.; Machimura, T.; et al. Stromal thrombospondin-1 expression is correlated with progression of esophagus squamous cell carcinoma. Anticancer Res 1999, 19, 4375–4378. [Google Scholar]
- Tenan, M.; Fulci, G.; Albertoni, M.; Diserens, A.C.; Hamou, M.F.; el Atifi-Borel, M.; Feige, J.J.; Pepper, M.S.; van Meir, E.G. Thrombospondin-1 is down regulated by anoxia and suppresses tumorgenicity of human glioblastoma cells. J. Exp. Med 2000, 191, 1789–1798. [Google Scholar]
- Henkin, J.; Volpert, O.V. Therapies using anti-angiogenic peptide mimetics of thrombospondin-1. Expert. Opin. Ther. Targets 2011, 15, 1369–1386. [Google Scholar]
- Grant, S.W.; Kyshtoobayeva, A.S.; Kurosaki, T.; Jakowatz, J.; Fruehauf, J.P. Mutant p53 correlates with reduced expression of thrombospondin-1, increasing angiogenesis and metastatic progression in melanoma. Cancer Detect. Prev 1998, 22, 185–194. [Google Scholar]
- Wu, M.P.; Tzeng, C.C.; Wu, L.W.; Huang, K.F.; Chou, C.Y. Thrombospondin-1 acts as a fence to inhibit angiogenesis that occurs during cervical carcinogenesis. Cancer J 2004, 10, 27–32. [Google Scholar]
- Byrne, G.J.; Hayden, K.E.; McDowell, G.; Lang, H.; Kirwan, C.C.; Tetlow, L.; Kumar, S.; Bundred, N.J. Angiogenic characteristics of circulating and tumoural thrombospondin-1 in breast cancer. Int. J. Oncol 2007, 31, 1127–1132. [Google Scholar]
- Nakao, T.; Kurita, N.; Komatsu, M.; Yoshikawa, K.; Iwata, T.; Utsunomiya, T.; Shimada, M. Expression of thrombospondin-1 and Ski are prognostic factors in advanced gastric cancer. Int. J. Clin. Oncol 2011, 16, 145–152. [Google Scholar]
- Tang, D.; Nagano, H.; Yamamoto, H.; Wada, H.; Nakamura, M.; Kondo, M.; Otam, H.; Yoshioka, S.; Kato, H.; Damdinsuren, B.; et al. Angiogenesis in cholangiocellular carcinoma: Expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significance. Oncol. Rep 2006, 15, 525–532. [Google Scholar]
- Rodriguez-Manzaneque, J.C.; Lane, T.F.; Ortega, M.A.; Hynes, R.O.; Lawler, J.; Iruela-Arispe, M.L. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc. Natl. Acad. Sci. USA 2001, 98, 12485–12490. [Google Scholar]
- Miyanaga, K.; Kato, Y.; Nakamura, T.; Matsumura, M.; Amaya, H.; Horiuchi, T.; Chiba, Y.; Tanaka, K. Expression and role of thrombospondin-1 in colorectal cancer. Anticancer Res 2002, 22, 3941–3948. [Google Scholar]
- Ioachim, E.; Damala, K.; Tsanou, E.; Briasoulis, E.; Papadiotis, E.; Mitselou, A.; Charhanti, A.; Doukas, M.; Lampri, L.; Arvantis, D.L. Thrombospondin-1 expression in breast cancer: Prognostic significance and association with p53 alterations, tumour angiogenesis and extracellular matrix components. Histol. Histopathol 2012, 27, 209–216. [Google Scholar]
- Tanaka, K.; Sonoo, H.; Kurebayashi, J.; Nomura, T.; Ohkubo, S.; Yamamoto, Y.; Yamamoto, S. Inhibition of infiltration and angiogenesis by thrombospondin-1 in papillary thyroid carcinoma. Clin. Cancer Res 2002, 8, 1125–1131. [Google Scholar]
- Wang, T.N.; Qian, X.-H.; Granick, M.S.; Solomon, M.P.; Rothman, V.L.; Berger, D.H.; Tuszynski, G.P. Thrombospondin-1 (TSP-1) promotes the invasive properties of human breast cancer. J. Surg. Res 1996, 63, 39–43. [Google Scholar]
- Sid, B.; Langlois, B.; Sartelet, H.; Bellon, G.; Dedieu, S.; Martiny, L. Thrombospondin-1 enhances human thyroid carcinoma cell invasion though urokinase activity. Int. J. Biochem. Cell Biol 2008, 40, 1890–1900. [Google Scholar]
- Yee, K.O.; Connolly, C.M.; Duquette, M.; Kazerounian, S.; Washington, R.; Lawler, J. The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res. Treat 2009, 114, 85–96. [Google Scholar]
- Sutton, C.D.; O’Byrne, K.; Goddard, J.C.; Marshall, L.J.; Jones, L.; Garcea, G.; Dennison, A.R.; Poston, G.; Lloyd, D.M.; Berry, D.P. Expression of thrombospondin-1 in resected colorectal liver metastases predicts poor prognosis. Clin. Cancer Res 2005, 11, 6567–6573. [Google Scholar]
- Firlej, V.; Mathieu, J.R.R.; Gilbert, C.; Lemonnier, L.; Nakhlé, J.; Gallou-Kabani, C.; Guarmit, B.; Morin, A.; Prevarskaya, N.; Delomgchamps, N.B.; et al. Thrombospondin-1 triggers cell migration and development of advanced prostate tumors. Cancer Res 2011, 71, 7649–7656. [Google Scholar]
- Maeda, K.; Nishiguchi, Y.; Kang, S.M.; Yashiro, M.; Onoda, N.; Sawada, T.; Ishikawa, T.; Hirakawa, K. Expression of thrombospondin-1 inversely correlated with tumor vascularity and hematogenous metastasis in colon cancer. Oncol. Rep 2001, 8, 763–766. [Google Scholar]
- Fleitas, T.; Martínez-sales, V.; Villa, V.; Reganon, E.; Mesado, D.; Martín, M.; Gómez-Cordina, J.; Montalar, J.; Reynés, G. VEGF and TSP1 correlate with prognosis in advanced non-small cell lung cancer. Clin. Transl. Oncol. 2013. [Google Scholar] [CrossRef]
- Rice, A.J.; Steward, M.A.; Quinn, C.M. Thrombospondin 1 protein expression relates to good prognostic indices in ductal carcinoma in situ of the breast. J. Clin. Pathol 2002, 55, 921–955. [Google Scholar]
- Poon, R.T.; Chung, K.K.; Cheung, S.T.; Lau, C.P.; Tong, S.W.; Leung, K.L.; Yu, W.C.; Tuszynski, G.P.; Fan, S.T. Clinical significance of thrombospondin 1 expression in hepatocellular carcinoma. Clin. Cancer Res 2004, 15, 4150–4157. [Google Scholar]
- Hawighorst, T.; Oura, H.; Streit, M.; Janes, L.; Nguyen, L.; Brown, L.F.; Oliver, G.; Jackson, D.G.; Detmar, M. Thrombospondin-1 selectively inhibits early-stage carcinogenesis and angiogenesis but not tumor lymphangiogenesis and lymphatic metastasis in transgenic mice. Oncogene 2002, 21, 7945–7956. [Google Scholar]
- Doll, J.A.; Reiher, F.K.; Crawford, S.E.; Pins, M.R.; Campbell, S.C.; Bouck, N.P. Thrombospondin-1, vascular endothelial growth factor and fibroblast growth factor-2 are key functional regulators of angiogenesis in the prostate. Prostate 2001, 49, 293–305. [Google Scholar]
- Fitchev, P.P.; Wcislak, S.M.; Lee, C.; Bergh, A.; Brendler, C.B.; Stellmach, V.M.; Crawford, S.E.; Marvroudis, C.D.; Cornwell, M.L.; Doll, J.A. Thrombospondin-1 regulates the normal prostate in vivo through angiogenesis and TGF-β activation. Lab. Invest 2010, 90, 1078–1090. [Google Scholar]
- Good, D.J.; Polverini, P.J.; Rastinejad, F.; Le, B.M.; Lemons, R.S.; Frazier, W.A.; Bouck, N.P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable form a fragment of thrombospondin. Proc. Natl. Acad. Sci. USA 1990, 87, 6624–6628. [Google Scholar]
- Jin, R.J.; Kwak, C.; Ree, S.G. The application of an antiangiogenic gene (thrombospondin-1) in the treatment of human prostate cancer xenograft model. Cancer Gene Ther 2000, 7, 1537–1542. [Google Scholar]
- Grossfeld, G.D.; Carroll, P.R.; Lindeman, N.; Meng, M.; Groshen, S.; Feng, A-C.; Hawes, D.; Cote, R.J. Thrombospndin-1 expression in patients with pathologic stage T3 prostate cancer undergoing radical prostatectomy: Association with p53 alterations, tumor angiogenesis, and tumor progression. Urology 2002, 59, 97–102. [Google Scholar]
- Colombel, M.; Filleur, S.; Fournier, P.; Merle, C.; Guglielmi, J.; Courtin, A.; Degeorges, A.; Serre, C.M.; Bouvier, R.; Clézardin, P.; et al. Androgens repress the expression of the angiogenesis inhibitor thrombospondin-1 in normal and neoplastic prostate. Cancer Res 2005, 65, 300–308. [Google Scholar]
- Kwak, C.; Jin, R.J.; Lee, C.; Park, M.S.; Lee, S.E. Thorombospondin-1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasia. BJU Int 2002, 89, 303–309. [Google Scholar]
- Vallbo, C.; Wang, W.; Damber, J.-E. The expression of thrombospondin-1 in benign prostatic hyperplasia and prostatic intraepithelial neoplasia is decreased in prostatic cancer. BJU Int 2004, 92, 1339–1343. [Google Scholar]
- Bastian, M.; Steiner, M.; Schuff-Werner, P. Expression of thrombospondin-1 in prostate-derived cell lines. Int. J. Mol. Med 2005, 15, 49–56. [Google Scholar]
- Kaygusuz, G.; Tulunay, O.; Baltaci, S.; Gogus, O. Microvessel density and regulators of angiogenesis in malignant and nonmalignant prostate tissue. Int. Urol. Nephrol 2007, 39, 841–850. [Google Scholar]
- Nelius, T.; Filleur, S.; Yemelyanov, A.; Budunov, A.; Budunoval, I.; Shroff, E.; Mirochnik, Y.; Aurora, A.; Veliceasa, D.; Xiao, W.; et al. Androgen receptor target NFkappaB and TSP1 to suppress prostate tumor growth in vivo. Int. J. Cancer 2007, 121, 999–1008. [Google Scholar]
- Goel, H.L.; Moro, L.; Murphy-Ullrich, J.E.; Hsieh, C.C.; Wu, C.L.; Jiang, Z.; Languino, L.R. Beta 1 integrin cytoplasmic variants differentially regulate expression of the antiangiogenic extracellular matrix protein thrombospondin 1. Cancer Res 2009, 69, 5374–5382. [Google Scholar]
- Mehta, R.; Kyshtoobayeva, A.; Kurosaki, T.; Small, E.J.; Kim, H.; Stroup, R.; McLaren, C.E.; Li, K.-T.; Fruehauf, J.P. Independent association of angiogenesis index with outcome in prostate cancer. Clin. Cancer Res 2001, 7, 81–88. [Google Scholar]
- Babiker, A.A.; Magnusson, P.U.; Ronquist, G.; Nilsson, B.; Ekdahl, K.N. Mapping pro- and antiangiogenic factors on the surface of proteasomes of normal and malignant cell origin. Prostate 2010, 70, 834–847. [Google Scholar]
- Miyata, Y.; Koga, S.; Takehara, K.; Kanetake, H.; Kanda, S. Expression of thrombospondin-derived 4N1K peptide-containing proteins in renal cell carcinoma tissues is associated with a decrease in tumor growth and angiogenesis. Clin. Cancer Res 2003, 9, 1734–1740. [Google Scholar]
- Klatte, T.; Böhm, M.; Nelius, T.; Filleur, S.; Reiher, F.; Allhoff, E.P. Evaluation of peri-operative peripheral and renal venous levels of pro- and anti-angiogenic factors and their relevance in patients with renal cell carcinoma. BJU Int 2007, 100, 209–214. [Google Scholar]
- Veliceasa, D.; Ivanovic, M.; Hoepfner, F.T.-S.; Thumbikat, P.; Volpert, O.V.; Smith, N.D. Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J 2007, 274, 6365–6377. [Google Scholar]
- Zubac, D.P.; Bostad, L.; Kihl, B.; Seidal, T.; Wentzel-Larsen, T.; Haukaas, S.A. The expression of thrombospondin-1 and p53 in clear cell renal cell carcinoma: Its relationship to angiogenesis, cell proliferation and cancer specific survival. J. Urol 2009, 182, 2144–2149. [Google Scholar]
- Zubac, D.P.; Wentzel-Larsen, T.; Seidal, T.; Bostad, L. Type 1 plasminogen activator inhibitor (PAI-1) in clear cell renal cell carcinoma (CCRCC) and its impact on angiogenesis, progression and patient survival after radical nephrectomy. BMC Cancer 2010, 10, 20–25. [Google Scholar]
- Baltaci, S.B.; Orhan, D.; Göğüş, Ç; Filiz, E.; Tulunay, Ö.; Göğüş, O. Thrombospondin-1, vascular endothelial growth factor expression and microvessel density in renal cell carcinoma and their relationship with multifocality. Eur. Urol 2003, 44, 76–81. [Google Scholar]
- Bienes-Martínez, R.; Ordóńz, A.; Feijoo-Cuaresma, M.; Corral-Escariz, M.; Mateo, G.; Stenina, O.; Jiménez, B.; Calzada, M.J. Autocrine stimulation of clear-cell renal cell carcinoma cell migration in hypoxia via HJF-independent suppression of thrombospondin-1. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef]
- Lee, Y.J.; Koch, M.; Karl, D.; Torres-Collado, A.X.; Fernando, N.T.; Rothrock, C.; Kuruppu, D.; Ryeom, S.; Iruela-Arispe, M.L.; Yoon, S.S. Variable inhibition of thrombospondin 1 against liver and lung metastases though differential activation of metalloproteinase ADAMTS1. Cancer Res 2010, 70, 948–956. [Google Scholar]
- Hassen, W.; Droller, M.J. Current concepts and treatment of bladder cancer. Curr. Opin. Urol 2000, 10, 291–299. [Google Scholar]
- Grossfeld, G.D.; Ginsberg, D.A.; Stein, J.P.; Bochner, B.H.; Esrig, D.; Groshen, S.; Dunn, M.; Nichols, P.W.; Taylor, C.R.; Skinner, D.G.; et al. Thrombospondin-1 expression in bladder cancer: Association with p53 alterations, tumor angiogenesis, and tumor progression. J. Natl. Cancer Inst 1997, 89, 219–227. [Google Scholar]
- Goddard, J.C.; Sutton, C.D.; Jones, J.L.; O’Byrne, K.J.; Kochelbergh, R.C. Reduced thrombospondin-1 at presentation predicts disease progression in superficial bladder cancer. Eur. Urol 2002, 42, 464–468. [Google Scholar]
- Ioachim, E.; Michael, M.C.; Salmas, M.; Damala, K.; tsanou, E.; Michael, M.M.; Malamou-Mitsi, V.; Stavropoulos, N.E. Thrombospondin-1 expression in urothelial carcinoma: Prognostic significance and association with p53 alterlations, tumour angiogenesis and extracellular matrix components. BMC Cancer 2006, 6, 140–147. [Google Scholar]
- Beecken, W.-D.; Engl, T.; Jonas, D.; Blaheta, R.A. Expression of angiogenesis inhibitors in human bladder cancer may explain rapid metastatic progression after radial cystectomy. Int. J. Mol. Med 2009, 23, 261–266. [Google Scholar]
- Johnson, A.M.; O’Connell, M.J.; Miyamoto, H.; Huang, J.; Yao, J.L.; Messing, E.M.; Reeder, J.E. Androgenic dependence of exophytic tumor growth in a transgenic mouse model of bladder cancer: A role for thrombospondin-1. BMC Urol. 2008, 8. [Google Scholar] [CrossRef]
- Byler, T.K.; Leocadio, D.; Shapiro, O.; Bratslavsky, G.; Stodgell, C.J.; Wood, R.W.; Messing, E.M.; Reeder, J.E. Valproic acid decreases urothelial cancer cell proliferation and induces thrombospondin-1 expression. BMC Urol. 2012, 12. [Google Scholar] [CrossRef]
- Donmez, G.; Sullu, Y.; Baris, S.; Yildiz, L.; Aydin, O.; Karagoz, F.; Kandemir, B. Vascular endothelial growth factor (VEGF), matrix metalloproteinase-9 (MMP-9), and thrombospondin-1 (TSP-1) expression in urothelial carcinomas. Pathol. Res. Pract 2009, 205, 854–857. [Google Scholar]
- Shariat, S.F.; Youssef, R.F.; Gupta, A.; Chade, D.C.; Karakiewicz, P.I.; Isbarn, H.; Jeldres, C.; Sagalowsky, A.I.; Ashfaq, R.; Lotan, Y. Associated of angiogenesis related markers with bladder cancer outcomes and other molecular markers. J. Urol 2010, 183, 1744–1750. [Google Scholar]
- Silverstein, R.; Harpel, P.C.; Nachman, R.L. Tissue plasminogen activator and urokinase enhances the binding of plasminogen to thrombospondin. J. Biol. Chem 1986, 261, 9959–9965. [Google Scholar]
- Bein, K.; Simons, M. Thrombospondin type I repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J. Biol. Chem 2000, 275, 32167–32173. [Google Scholar]
- Emonard, H.; Bellon, G.; Troeberg, L.; Berton, A.; Robinet, A.; Henriet, P.; Marbaix, E.; Kirkegaard, K.; Patthy, L.; Eeckhout, Y.; et al. Low density lipoprotein receptor-related protein mediates endocytic clearance of pro-MMP-2·TIMP-2 complex through a thrombospondin-independent mechanism. J. Biol. Chem 2004, 279, 54944–24951. [Google Scholar]
- Orr, A.W.; Elzie, C.A.; Kucik, D.F.; Murphy-Ullrich, J.E. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J. Cell Sci 2003, 116, 2917–2927. [Google Scholar]
- John, A.S.; Hu, X.; Rothman, V.L.; Tuszynski, G.P. Thrombospondin-1 (TSP-1) up-regulates tissue inhibitor of metalloproteinase-1 (TIMP-1) production in human tumor cells: Exploring the functional significance in tumor cell invasion. Exp. Mol. Pathol 2009, 87, 184–188. [Google Scholar]
- Soff, G.A.; Sanderowitz, J.; Gately, S.; Verrusio, E.; Weiss, I.; Berm, S.; Kwaan, H.C. Expression of plasminogen activator inhibitor type 1 by human prostate carcinoma cells inhibits primary tumor growth, tumor-associated angiogenesis. J. Clin. Invest 1995, 96, 2593–2600. [Google Scholar]
- Ohba, K.; Miyata, Y.; Kanda, S.; Koga, S.; Hayashi, T.; Kanetake, H. Expression of urokinase-type plasminogen activator, urokinase-type plasminogen activator recptor and plasminogen activator inhibitors in patients with renal cell carcinoma: Correlation with tumor associated macrophage and prognosis. J. Urol 2005, 174, 461–465. [Google Scholar]
- Demeron, K.M.; Volpert, O.V.; Tainsky, M.A.; Bouck, N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 1994, 265, 1582–1584. [Google Scholar]
- Tokunaga, T.; Nakamura, M.; Oshika, Y.; Tsuchida, T.; Kazuno, M.; Fukushima, Y.; Kawai, K.; Abe, Y.; Kijima, H.; Yamazaki, H.; et al. Alterations in tumor suppressor gene p53 corelate with inhibition of thrombosponsin-1 gene expression in colon cancer cells. Virchow. Arch 1998, 433, 415–433. [Google Scholar]
- Kawahara, N.; Ono, M.; Taguchi, K.; Okamoto, M.; Shimada, M.; Takenaka, K.; Hayashi, K.; Mosher, D.F.; Sugimachi, K.; Tsuneyoshi, M.; et al. Enhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinoma. Hepatology 1998, 28, 1512–1517. [Google Scholar]
- Kazerounian, S.; Yee, K.O.; Lawler, J. Thrombospondins in cancer. Cell. Mol. Life Sci 2008, 65, 700–712. [Google Scholar]
- Scarpino, S.; Di Napoli, A.; Taraboletti, G.; Cancrini, A.; Ruco, L.P. Hepatocyte growth factor (HGF) downregulates thrombospondin 1 (TSP-1) expression in thyroid papillary carcinoma cells. J. Pathol 2005, 205, 50–56. [Google Scholar]
- Wei, W.; Kong, B.; Qu, X. Alteration of HGF and TSP-1 expression in ovarian carcinoma associated with clinical features. J. Obstet. Gynaecol. Res 2012, 38, 57–64. [Google Scholar]
- Wei, W.; Kong, B.; Yang, Q.; Qu, X. Hepatocyte growth factor enhances ovarian cancer cell invasion through down-regulation of thrombospondin-1. Cancer Biol. Ther 2010, 9, 79–87. [Google Scholar]
- Weber, A.; Kristiansen, I.; Johannsen, M.; Oelrich, B.; Scholmann, K.; Gunia, S.; May, M.; Meyer, H.A.; Behnke, S.; Moch, H.; et al. The FUSE binding proteins FBP1 and FBP3 are potential c-myc regulators in renal, but not in prostate and bladder cancer. BMC Cancer 2008, 8. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.W.; Chang, W.H.; Su, Y.C.; Chen, Y.C.; Lai, Y.H.; Wu, P.T.; Hsu, C.I.; Lin, W.C.; Lai, M.K.; Lin, J.Y. MYC pathway is activated in clear cell renal cell carcinoma and essential for proliferation of clear cell renal cell carcinoma cells. Cancer Lett 2009, 273, 35–43. [Google Scholar]
- Jenkins, R.B.; Qian, J.; Lieber, M.M.; Bostwick, D.G. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res 1997, 57, 524–531. [Google Scholar]
- Zaharieva, B.; Simon, R.; Ruiz, C.; Oeggerli, M.; Mihatsch, M.J.; Gasser, T.; Sauter, G.; Toncheva, D. High-throughput tissue microarray analysis of CMYC amplificationin urinary bladder cancer. Int. J. Cancer 2005, 117, 952–956. [Google Scholar]
- Yamamura, S.; Saini, S.; Majid, S.; Hirata, H.; Ueno, K.; Chang, I.; Tanaka, Y.; Gupta, A.; Dahiya, R. MicroRNA-34a suppresses malignant transformation by targeting c-Myc transcriptional complexes in human renal cell carcinoma. Carcinogenesis 2012, 33, 294–300. [Google Scholar]
- Yamada, Y.; Hidaka, H.; Seki, N.; Yoshino, H.; Yamasaki, T.; Itesako, T.; Nakagawa, M.; Enokida, H. Tumor-suppressive microRNA-135a inhibits cancer cell proliferation by targeting the c-MYC oncogene in renal cell carcinoma. Cancer Sci 2013, 104, 304–312. [Google Scholar]
- Tikhonenko, A.T.; Black, D.J.; Linial, M.L. Viral Myc oncoproteins in infected fibroblasts down-modulate thrombospondin-1, a possible tumor suppressor gene. J. Biol. Chem 1996, 271, 30741–30747. [Google Scholar]
- Janz, A.; Sevignani, C.; Kenyon, K.; Ngo, C.V.; Thomas-Tikhonenko, A. Activation of the myc oncoprotein leads to increased turnover of thrombospondin-1 mRNA. Nucleic Acids Res 2000, 28, 2268–2275. [Google Scholar]
- Zhou, L.; Picard, D.; Ra, Y.S.; Li, M.; Northcott, P.A.; Hu, Y.; Stearns, D.; Hawkins, C.; Taylor, M.D.; Rutka, J.; et al. Silencing of thrombospondin-1 is critical for myc-induced metastatic phenotypes in medulloblastoma. Cancer Res 2010, 70, 8199–8210. [Google Scholar]
- Zabrenetzky, V.; Harris, C.C.; Steeg, P.S.; Roberts, D.D. Expression of the extracellular matrix molecule thrombospondin inversely correlates with malignant progression in melanoma, lung, and breast carcinoma cell lines. Int. J. Cancer 1994, 59, 191–195. [Google Scholar]
- Weinstat-Saslow, D.L.; Zabrenetzky, V.S.; VanHoutte, K.; Fraizier, W.A.; Roberts, D.D.; Steeg, P.S. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res 1994, 54, 6504–6511. [Google Scholar]
- Mettouchi, A.; Cabon, F.; Montreau, N.; Vernier, P.; Mercier, G.; Blangy, D.; Tricoire, H.; Vigier, P.; Binétruy, B. SPARC and thrombospondin genes are repressed by c-jun oncogene in rat emryo fibroblasts. EMBO J 1994, 13, 5668–5678. [Google Scholar]
- Moon, Y.; Bottone, F.G.; McEntee, M.F.; Gahtan, V. Suppression of tumour cell invasion by cyclooxygenase inhibitors is mediated by thrombospondin-1 via the early growth response gene Egr-1. Mol. Cancer Ther 2005, 5, 1551–1558. [Google Scholar]
- Ren, B.; Yee, K.O.; Lawler, J.; Khosravi-Far, R. Regulation of thrombospondin-1. Biochem. Biophys. Acta 2006, 1765, 178–188. [Google Scholar]
- Su, F.; Pascal, L.E.; Xiao, W.; Wang, Z. Tumor suppressor U19/EAF2 regulates thrombospondin-1 expression via p53. Oncogene 2010, 29, 421–431. [Google Scholar]
- Wang, Z.; Tufts, R.; Haleem, R.; Cai, X. Genes regulated by androgen in the rate ventral prostate. Proc. Natl. Acad. Sci. USA 1997, 94, 12999–13004. [Google Scholar]
- Xiao, W.; Zhang, Q.; Jiang, F.; Pins, M.; Kozlowski, J.M.; Wang, Z. Suppression of prostate tumor growth by U19, a novel testosterone-regulated apoptotic inducer. Cancer Res 2003, 63, 4698–4704. [Google Scholar]
- Kaur, S.; Martin-Manso, G.; Pendrak, M.L.; Garfield, S.H.; Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J. Biol. Chem 2010, 285, 38923–38932. [Google Scholar]
- Guo, N.-H.; Krutzsch, H.C.; Inman, J.K.; Robert, D.D. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induced apoptosis of endothelial cells. Cancer Res 1997, 57, 1735–1742. [Google Scholar]
- Bruel, A.; Touhami-Carrier, A.; Thomaidis, A.; Legrand, C. Thrombospondin-1 (TSP-1) and TSP-1-derived heparin-binding peptides induces promyelocytic leukemia cell differentiation and apoptosis. Anticancer Res 2005, 25, 757–764. [Google Scholar]
- Tolsma, S.S.; Volpert, O.V.; Good, D.J.; Frazier, W.A.; Polverini, P.J.; Bouck, N.P. Peptodes derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J. Cell Biol 1993, 122, 497–511. [Google Scholar]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochem. Biophys. Acta 2007, 1772, 937–946. [Google Scholar]
- Saumet, A.; Slimane, B.M.; Lanotte, M.; Lawler, J.; Dubermrd, V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death though CD47/αvβ3 in promyelocytic leukemia NB4 cells. Blood 2005, 106, 658–667. [Google Scholar]
- De Fraipont, F.; Keramidas, M.; el Atifti, M.; Chambaz, E.M.; Berger, F.; Feige, J.-J. Expression of thrombospondin 1 fragment 167–569 in C6 glioma cells stimulates tumorgenecity despite reduced neovascularization. Oncogene 2004, 23, 3642–3649. [Google Scholar]
- Manna, P.P.; Frazier, W.A. CD47 mediates killing of breast tumor cell via Gi-dependent inhibitor of protein kinase A. Cancer Res 2004, 64, 1026–1036. [Google Scholar]
- Zhang, X.; Lawler, J. Thrombospondin-based antiangiogenic therapy. Microvasc. Res 2007, 74, 90–99. [Google Scholar]
- Markovic, S.N.; Suman, V.J.; Rao, R.A.; Ingle, J.N.; Kaur, J.S.; Erickson, L.A.; Pitot, H.C.; Croghan, G.A.; McWilliams, R.R.; Merchan, J.; et al. A phase II study of ABT-510 (thrombospondin-1 analogue) for the treatment of metastatic melanoma. Am. J. Clin. Oncol 2007, 30, 303–309. [Google Scholar]
- Baker, L.H.; Rowinsky, E.K.; Mendelson, D.; Humerickhouse, R.A.; Knight, R.A.; Qian, J.; Carr, R.A.; Gordon, G.B.; Demetri, G.D. Randomized phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor abt-510 in patients with advanced soft tissue sarcoma. J. Clin. Oncol 2008, 26, 5583–5588. [Google Scholar]
- Yap, R.; Veliceasa, D.; Emmenegger, U.; Kerbel, R.S.; McKay, L.M.; Henkin, J.; Volpert, O.V. Metronomic low-dose chemotherapy boots CD-95-dependent antiangiogenic effect of thrombospondin peptide ABT-510: A complementation antiangiogenic strategy. Clin. Cancer Res 2005, 15, 6678–6685. [Google Scholar]
- Haviv, F.; Bradley, M.F.; Kalvin, D.M.; Schneider, A.J.; Davidson, D.J.; Majest, S.M.; McKay, L.M.; Haskell, C.J.; Bell, R.L.; Nguyen, B.; et al. Thrombospondin-1 mimetic peptide inhibitors of angiogenesis and tumor growth: Design, synthesis, and optimization of pharmacokinetics and biological activities. J. Med. Chem 2005, 48, 2838–2846. [Google Scholar]
- Ebbinghaus, S.; Hussain, M.; Tannir, N.; Gordon, M.; Desai, A.A.; Knight, R.A.; Humerickhouse, R.A.; Quian, J.; Gordon, G.B.; Fliglin, R. Phase 2 study of ABT-510 in patients with previouslu untreated advanced renal cell carcinoma. Clin. Cancer Res 2007, 13, 6689–6695. [Google Scholar]
- Dalrymple, S.L.; Becker, R.E.; Issacs, J.T. The quinolone-3-carboxamaide anti-angiogenic agents, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts. Prostate 2007, 67, 790–797. [Google Scholar]
- Bratt, O.; Haggman, M.; Ahlgren, G.; Nordle, O.; Bjork, A.; Damber, J.E. Open-rabel, clinical phase I studies of tasquinimod in patients with castration-resistant prostate cancer. Br. J. Cancer 2009, 101, 1233–1240. [Google Scholar]
- Olsson, A.; Bjork, A.; Vallon-Christersson, J.; Issacs, J.T.; Leanderson, T. Tasquinimod (ABR-215050), a quinolone-3-carboxamide anti-angiogenic agents, modulates the expression of thrombospondin-1 in human prostate tumors. Mol. Cancer 2010, 9. [Google Scholar] [CrossRef]
- Jennbacken, K.; Welén, K.; Olsson, A.; Axelsson, B.; Törngren, M.; Damber, J.E.; Leanderson, T. Inhibition of metastasis in a castration resistant prostate cancer model by the quinolone-3-carboxamide tasquinimod (ABR-215050). Prostate 2012, 72, 913–924. [Google Scholar]
- Soto-Pantoja, D.R.; Ridnour, L.A.; Wink, D.A.; Roberts, D.D. Blockade of CD47 increases survival of mice exposed to lethal total body irradiation. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Maxhimer, J.B.; Hyodo, F.; Pendrak, M.L.; Ridnour, L.A.; DeGraff, W.G.; Tsokos, M.; Wink, D.A.; Roberts, D.D. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am. J. Pathol 2008, 173, 1100–1112. [Google Scholar]
- Maxhimer, J.B.; Soto-Pantoja, D.R.; Ridnour, L.A.; Shih, H.B.; Degraff, W.G.; Tsokos, M.; Wink, D.A.; Isenberg, J.S.; Roberts, D.D. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci. Transl. Med. 2009, 1. [Google Scholar] [CrossRef]
- Rath, G.M.; Schneider, C.; Dedieu, S.; Rothhut, B.; Soula-Rothhut, M.; Ghoneim, C.; Sid, C.B.; Morjani, H.; el Btaouri, H.; Martiny, L. The C-terminal CD47/IAP-binding domain of thrombospondin-1 prevents camptothecin- and doxorubicin-induced apoptosis in human thyroid carcinoma cells. Biochem. Biophys. Acta 2006, 1763, 1125–1134. [Google Scholar]
- Kaur, S.; Soto-Pantoja, D.R.; Stein, E.V.; Liu, C.; Elkahloun, A.G.; Pendrak, M.L.; Nicolae, A.; Singh, S.P.; Nie, Z.; Levens, D.; et al. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Uno, S.; Kinoshita, Y.; Azuma, Y.; Tsunenari, T.; Yoshimura, Y.; Iida, S.; Kikuchi, Y.; Yamada-Okabe, H.; Fukushima, N. Antitumor activity of a monoclonal antibody against CD47 in xenograft models of human leukemia. Oncol. Rep 2007, 17, 1189–1194. [Google Scholar]
- Reiher, F.K.; Volpert, O.V.; Jimenez, B.; Crawford, S.E.; Dinney, C.P.; Henkin, J.; Haviv, F.; Bouck, N.P.; Campbell, S.C. Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. Int. J. Cancer 2002, 98, 682–689. [Google Scholar]
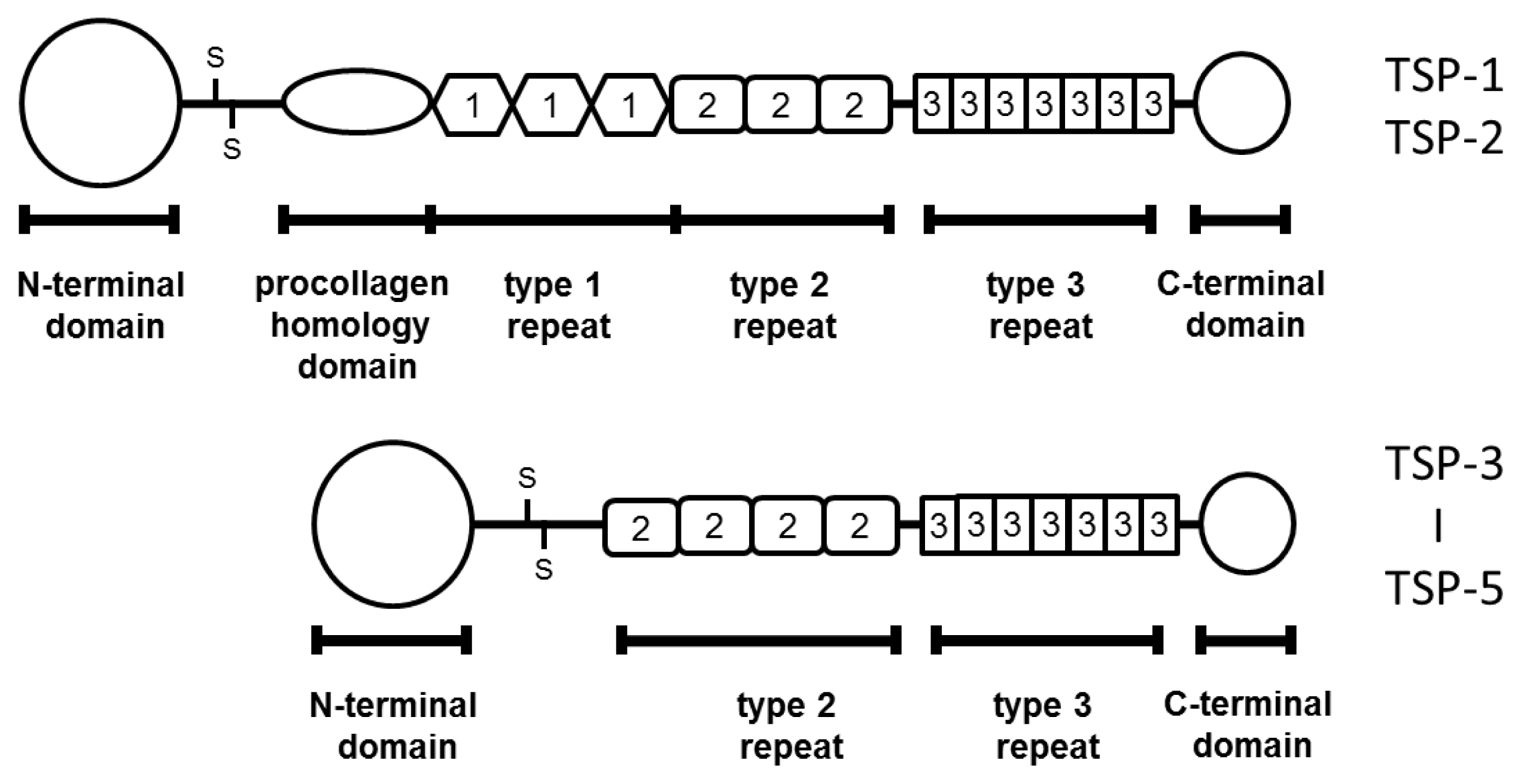
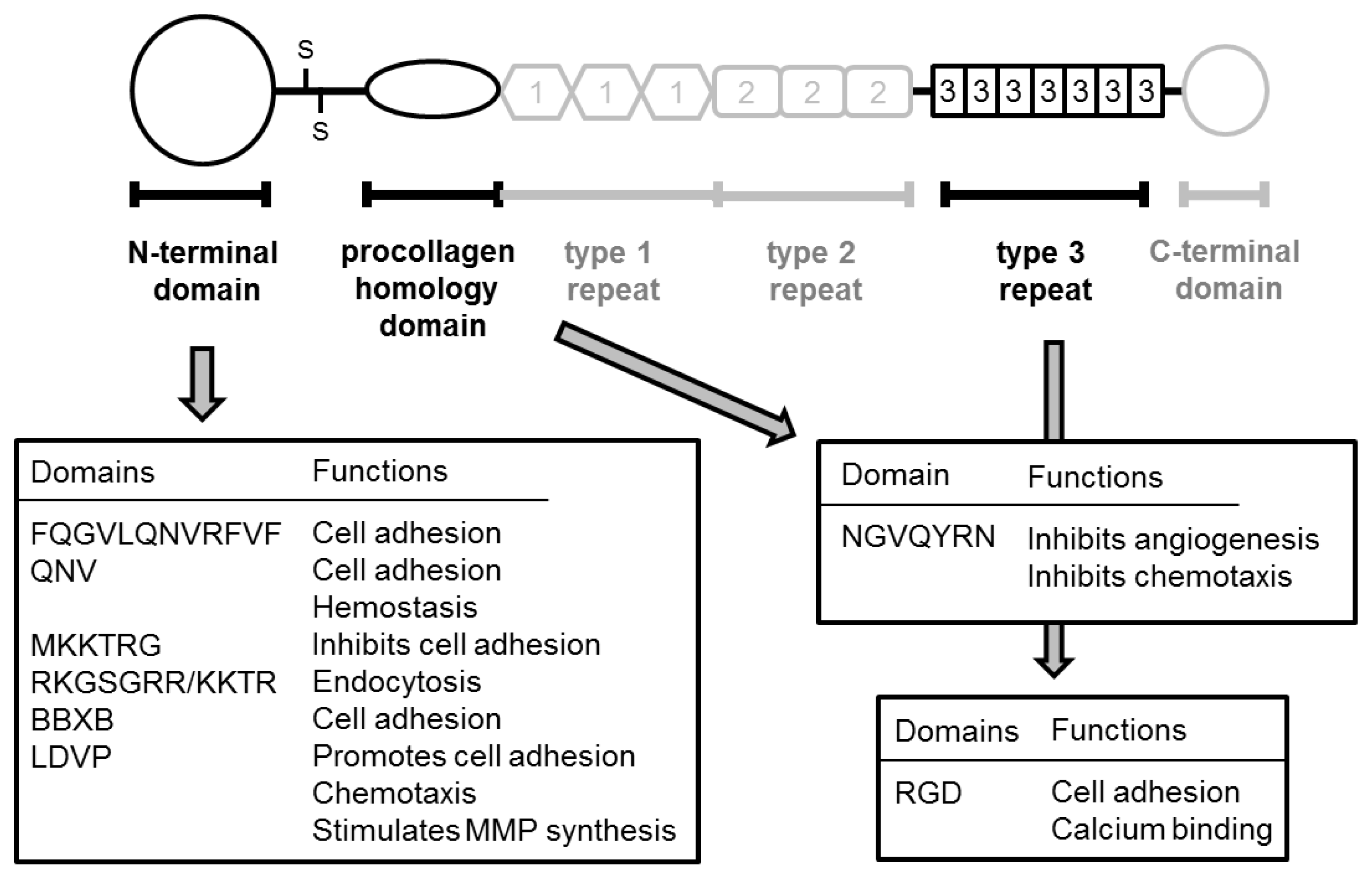
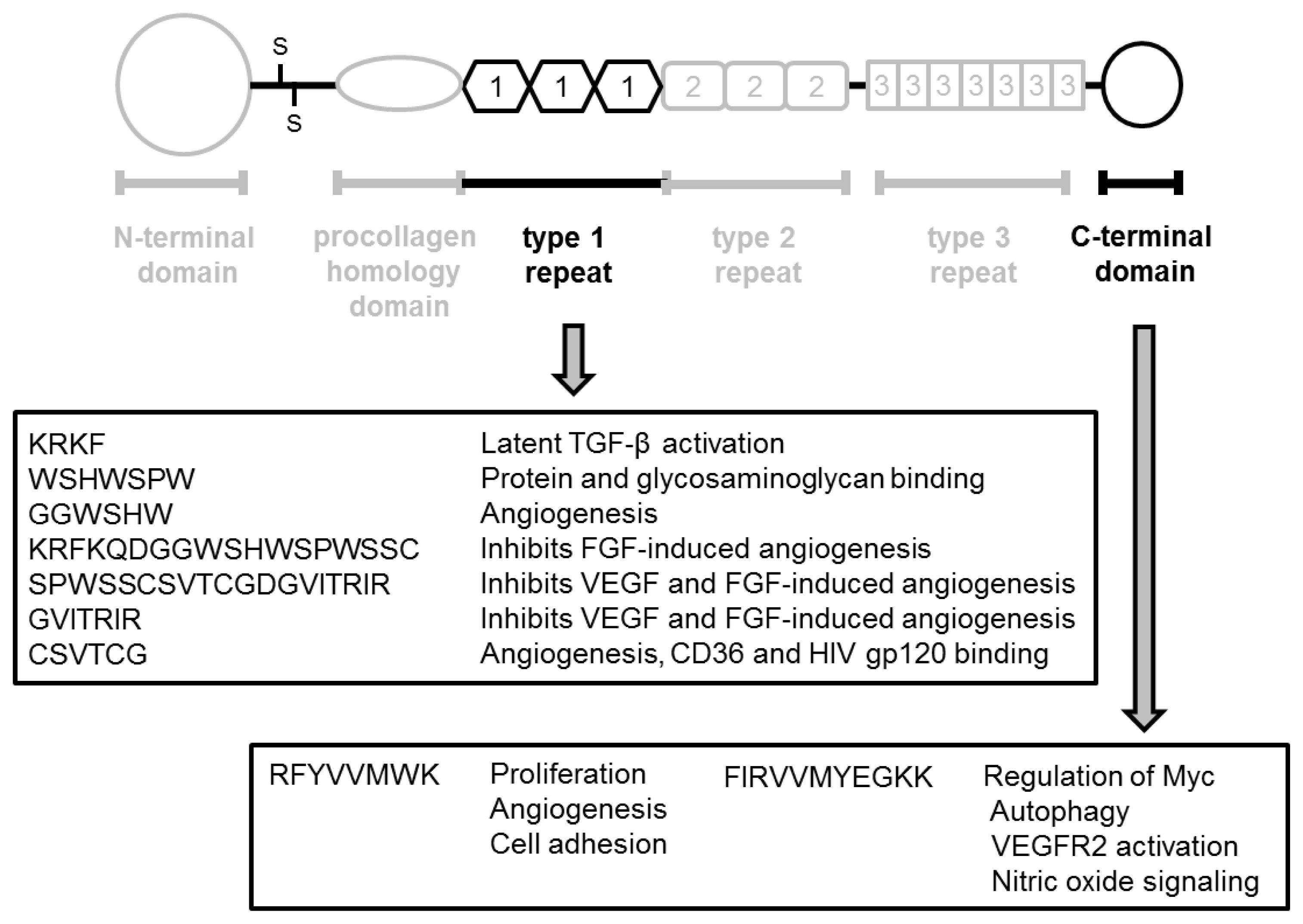
| Year | N | Method | Change * | GS | pT stage | Metastasis | Prognosis | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2001 | 98 | p-IHC | – | Neg. | – | – | Not S: survival | [69] |
| 2002 | 85 | p-IHC | – | Not S | – | – | Not S: survival | [61] |
| 2002 | 82 | p-IHC | Decrease | Not S | – | Neg. | – | [63] |
| 2004 | 34 | p-IHC | Decrease | – | – | – | – | [64] |
| 2005 | 60 | p-IHC | – | Neg. | – | – | – | [62] |
| 2007 | 55 | p-IHC | Increase | Not S | – | – | – | [66] |
| 2011 | 35 | RT-PCR ** | – | Not S | Pos. | – | PSA relapse | [51] |
| 2011 | 35 | RT-PCR *** | – | Not S | Not S | – | Not S | [51] |
| Year | N | Method | Change * | Grade | TNM stage | pT stage | Metastasis | Prognosis | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 2003 | 119 | p-IHC | – | – | – | Not S | Not S | – | [71] |
| 2007 | 74 | blood | – | Not S | Not S | – | – | Not S | [72] |
| 2007 | 17 | p-IHC | Not change | Neg. | – | – | – | – | [73] |
| 2009 | 172 | p-IHC | – | Neg. | Neg. | – | – | For survival | [74] |
| Year | N | Method | Change * | Grade | Stage | pT stage | Metastasis | Prognosis | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1997 | 163 | p-IHC | – | Not S | Not S | – | Not S | For survival | [80] |
| 2002 | 220 | p-IHC | – | Not S | Not S | – | – | For survival ** | [81] |
| 2006 | 148 | p-IHC *** | – | Neg. | – | Neg. | – | – | [82] |
| 2006 | 148 | p-IHC **** | – | Not S | – | Neg. | – | – | [82] |
| 2008 | 10 | WB | Decrease | [84] | |||||
| 2009 | 131 | p-IHC | – | Neg. | – | Neg. | Not S | – | [86] |
| 2010 | 204 | p-IHC | – | – | Neg. | – | Neg. for LN | For survival | [87] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Miyata, Y.; Sakai, H. Thrombospondin-1 in Urological Cancer: Pathological Role, Clinical Significance, and Therapeutic Prospects. Int. J. Mol. Sci. 2013, 14, 12249-12272. https://doi.org/10.3390/ijms140612249
Miyata Y, Sakai H. Thrombospondin-1 in Urological Cancer: Pathological Role, Clinical Significance, and Therapeutic Prospects. International Journal of Molecular Sciences. 2013; 14(6):12249-12272. https://doi.org/10.3390/ijms140612249
Chicago/Turabian StyleMiyata, Yasuyoshi, and Hideki Sakai. 2013. "Thrombospondin-1 in Urological Cancer: Pathological Role, Clinical Significance, and Therapeutic Prospects" International Journal of Molecular Sciences 14, no. 6: 12249-12272. https://doi.org/10.3390/ijms140612249




