Ectopic Overexpression of an AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) Gene OsIAA4 in Rice Induces Morphological Changes and Reduces Responsiveness to Auxin
Abstract
:1. Introduction
2. Results
2.1. Identification of OsIAA4
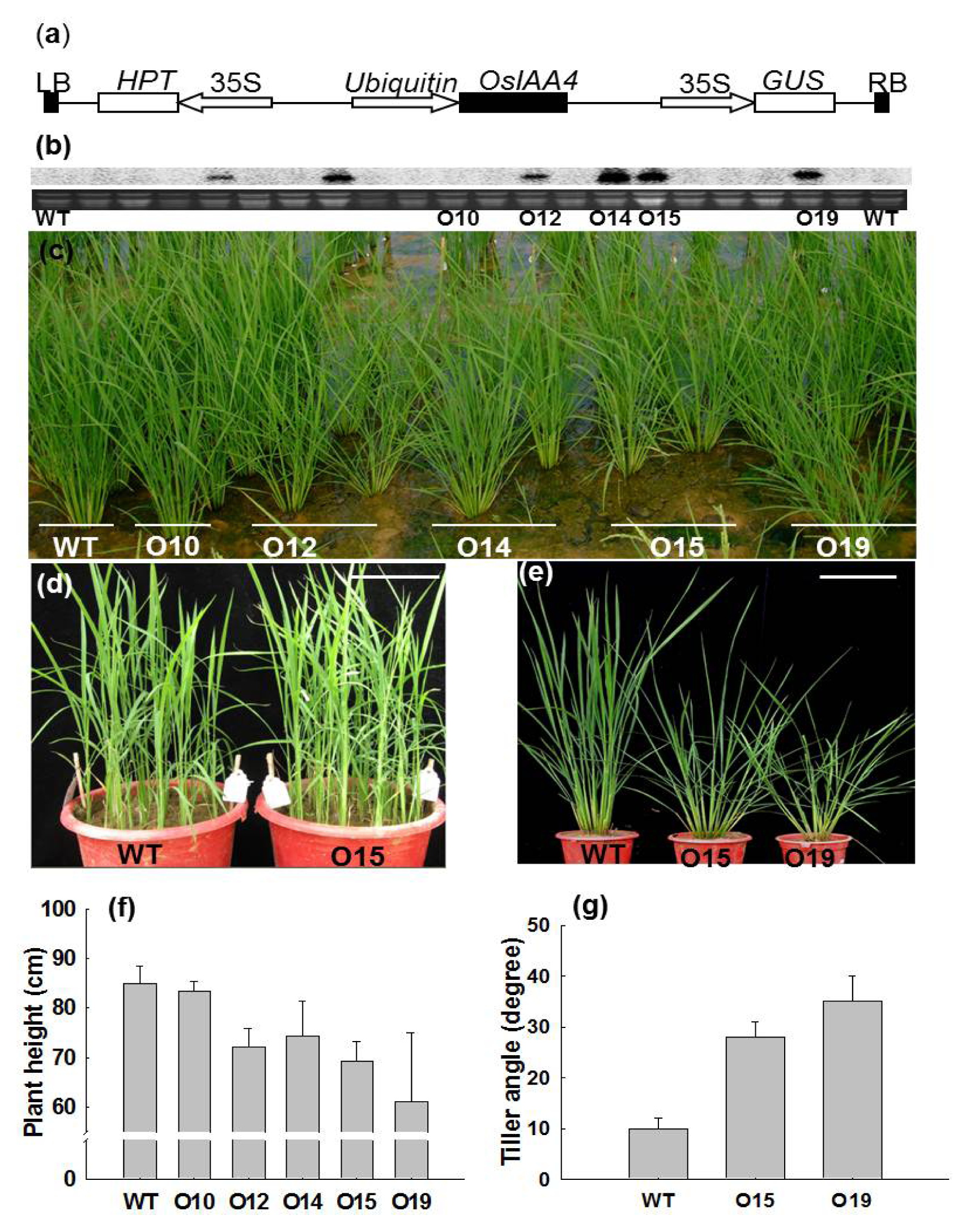
2.2. Morphological Changes of OsIAA4-Overexpressed Rice Plants
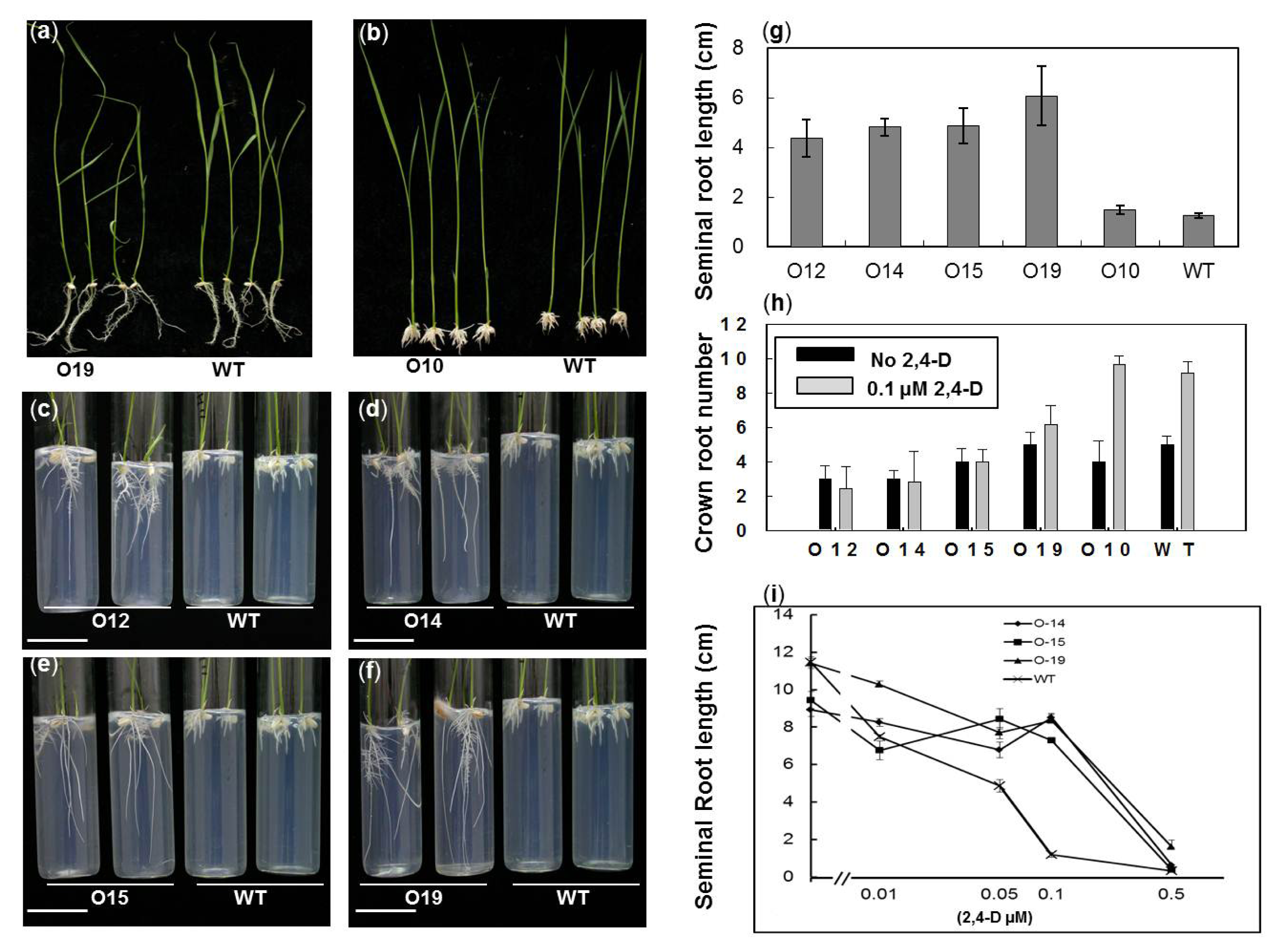
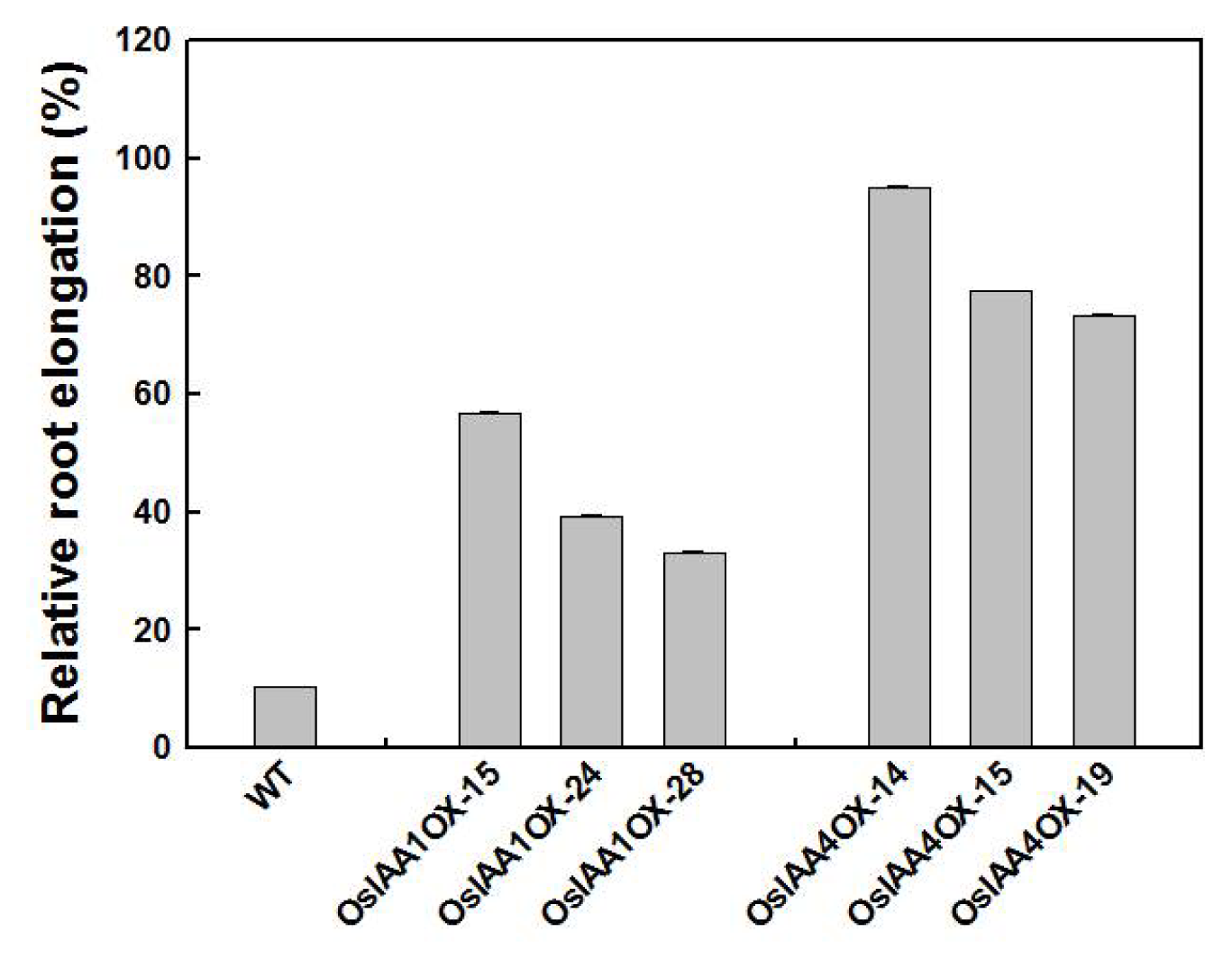
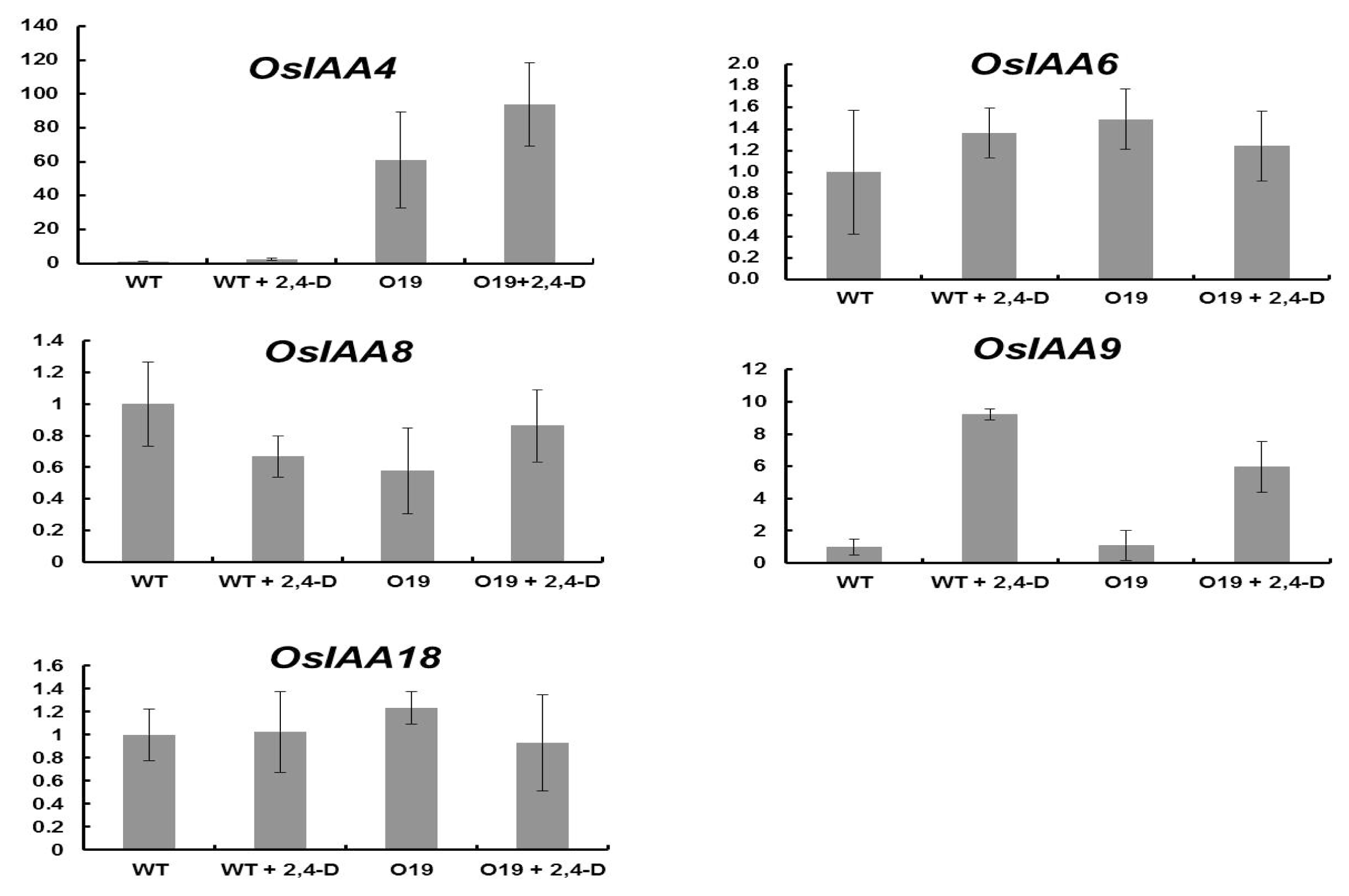
2.3. OsIAA4-Overexpressing Plants Show Less Sensitive to Auxin
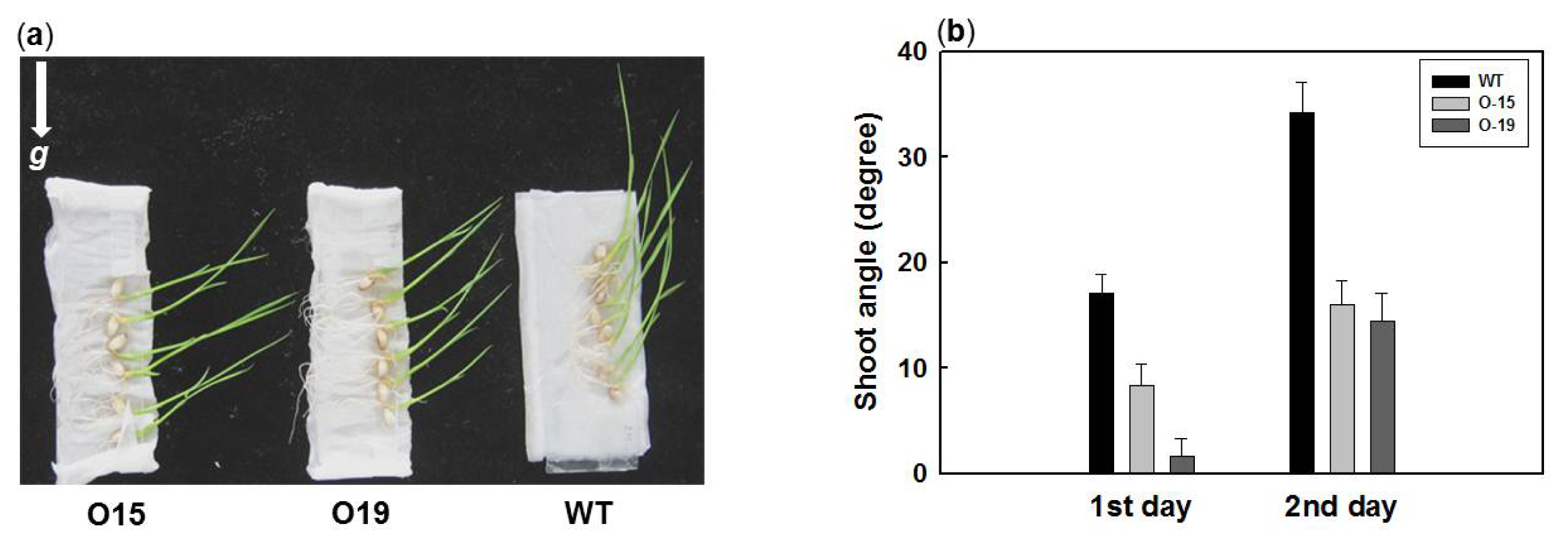
2.4. Overexpression of OsIAA4 Impaired Gravity Response in Shoot
3. Discussion
4. Materials and Methods
4.1. Construct and Rice Transformation
4.2. Northern Blot and Real-Time PCR
4.3. Plant Growth and Treatments
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Friml, J. Auxin transport—Shaping the plant. Curr. Opin. Plant. Biol 2003, 6, 7–12. [Google Scholar]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T.J. Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 2004, 16, 533–543. [Google Scholar]
- Gray, W.M.; Kepinski, S.; Rouse, D.; Leyser, O.; Estelle, M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 2001, 414, 271–276. [Google Scholar]
- Ramos, J.A.; Zenser, N.; Leyser, O.; Callis, J. Rapid degradation of auxin/indoleacetic acid proteins requiresconserved amino acids of domain II and is proteasome dependent. Plant Cell 2001, 13, 2349–2360. [Google Scholar]
- Kim, J.; Harter, K.; Theologis, A. Protein–protein interactions among the Aux/IAA proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 11786–11791. [Google Scholar]
- Ouellet, F.; Overvoorde, P.J.; Theologis, A. IAA17/AXR3: Biochemical insight into an auxin mutant phenotype. Plant Cell 2001, 13, 829–841. [Google Scholar]
- Yang, X.; Lee, S.; So, J.H.; Dharmasiri, S.; Dharmasiri, N.; Ge, L.; Jensen, C.; Hangarter, R.; Hobbie, L.; Estelle, M. The IAA1 protein is encoded by AXR5 and is a substrate of SCFTIR1. Plant. J. Cell Mol. Biol 2004, 40, 772–782. [Google Scholar]
- Tian, Q.; Reed, J.W. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 1999, 126, 711–721. [Google Scholar]
- Kim, B.C.; Soh, M.C.; Kang, B.J.; Furuya, M.; Nam, H.G. Two dominant photomorphogenic mutations of Arabidopsis thaliana identified as suppressor mutations of hy2. Plant J. Cell Mol. Biol 1996, 9, 441–56. [Google Scholar]
- Nagpal, P.; Walker, L.M.; Young, J.C.; Sonawala, A.; Timpte, C.; Estelle, M.; Reed, J.W. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol 2000, 123, 563–574. [Google Scholar]
- Hamann, T.; Benkova, E.; Baurle, I.; Kientz, M.; Jurgens, G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev 2002, 16, 1610–1615. [Google Scholar]
- Fukaki, H.; Tameda, S.; Masuda, H.; Tasaka, M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. Cell Mol. Biol 2002, 29, 153–168. [Google Scholar]
- Leyser, H.M.; Pickett, F.B.; Dharmasiri, S.; Estelle, M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. Cell Mol. Biol 1996, 10, 403–413. [Google Scholar]
- Reed, J.W. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant. Sci 2001, 6, 420–425. [Google Scholar]
- Uehara, T.; Okushima, Y.; Mimura, T.; Tasaka, M.; Fukaki, H. Domain II mutations in CRANE/IAA18 suppress lateral root formation and affect shoot development in Arabidopsis thaliana. Plant Cell Physiol 2008, 49, 1025–1038. [Google Scholar]
- Tatematsu, K.; Kumagai, S.; Muto, H.; Sato, A.; Watahiki, M.K.; Harper, R.M.; Liscum, E.; Yamamoto, K.T. MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 2004, 16, 379–393. [Google Scholar]
- Rogg, L.E.; Lasswell, J.; Bartel, B. A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell 2001, 13, 465–480. [Google Scholar]
- Nakamura, A.; Umemura, I.; Gomi, K.; Hasegawa, Y.; Kitano, H.; Sazuka, T.; Matsuoka, M. Production and characterization of auxin-insensitive rice by overexpression of a mutagenized rice IAA protein. Plant J. Cell Mol. Biol 2006, 46, 297–306. [Google Scholar]
- Kitomi, Y.; Inahashi, H.; Takehisa, H.; Sato, Y.; Inukai, Y. OsIAA13-mediated auxin signaling is involved in lateral root initiation in rice. Plant Sci. Int. J. Exp. Plant Biol 2012, 190, 116–122. [Google Scholar]
- Zhu, Z.X.; Liu, Y.; Liu, S.J.; Mao, C.Z.; Wu, Y.R.; Wu, P. A gain-of-function mutation in OsIAA11 affects lateral root development in rice. Mol. Plant 2012, 5, 154–161. [Google Scholar]
- Jun, N.; Gaohang, W.; Zhenxing, Z.; Huanhuan, Z.; Yunrong, W.; Ping, W. OsIAA23-mediated auxin signaling defines postembryonic maintenance of QC in rice. Plant J. Cell Mol. Biol 2011, 68, 433–442. [Google Scholar]
- Song, Y.; Wang, L.; Xiong, L. Comprehensive expression profiling analysis of OsIAA gene family in developmental processes and in response to phytohormone and stress treatments. Planta 2009, 229, 577–591. [Google Scholar]
- Song, Y.; You, J.; Xiong, L. Characterization of OsIAA1 gene, a member of rice Aux/IAA family involved in auxin and brassinosteroid hormone responses and plant morphogenesis. Plant Mol. Biol 2009, 70, 297–309. [Google Scholar]
- Wang, L.; Xie, W.B.; Chen, Y.; Tang, W.J.; Yang, J.Y.; Ye, R.J.; Liu, L.; Lin, Y.J.; Xu, C.G.; Xiao, J.H.; et al. A dynamic gene expression atlas covering the entire life cycle of rice. Plant J 2010, 61, 752–766. [Google Scholar]
- Park, J.Y.; Kim, H.J.; Kim, J. Mutation in domain II of IAA1 confers diverse auxin-related phenotypes and represses auxin-activated expression of Aux/IAA genes in steroid regulator-inducible system. Plant J. Cell Mol. Biol 2002, 32, 669–683. [Google Scholar]
- Tian, Q.; Uhlir, N.J.; Reed, J.W. Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 2002, 14, 301–319. [Google Scholar]
- Li, P.; Wang, Y.; Qian, Q.; Fu, Z.; Wang, M.; Zeng, D.; Li, B.; Wang, X.; Li, J. LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res 2007, 17, 402–410. [Google Scholar]
- Wu, X.; Tang, D.; Li, M.; Wang, K.; Cheng, Z. Loose plant architecture1, an INDETERMINATE DOMAIN protein involved in shoot gravitropism, regulates plant architecture in rice. Plant Physiol 2013, 161, 317–329. [Google Scholar]
- Yoshihara, T.; Iino, M. Identification of the gravitropism-related rice gene LAZY1 and elucidation of LAZY1-dependent and -independent gravity signaling pathways. Plant Cell Physiol 2007, 48, 678–688. [Google Scholar]
- Sato, A.; Yamamoto, K.T. Overexpression of the non-canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol. Plant 2008, 133, 397–472. [Google Scholar]
- Li, X.; Qian, Q.; Fu, Z.; Wang, Y.; Xiong, G.; Zeng, D.; Wang, X.; Liu, X.; Teng, S.; Hiroshi, F.; et al. Control of tillering in rice. Nature 2003, 422, 618–621. [Google Scholar]
- Sakamoto, T.; Morinaka, Y.; Ohnishi, T.; Sunohara, H.; Fujioka, S.; Ueguchi-Tanaka, M.; Mizutani, M.; Sakata, K.; Takatsuto, S.; Yoshida, S.; et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol 2006, 24, 105–109. [Google Scholar]
- Wang, Y.; Li, J. The plant architecture of rice (Oryza sativa). Plant Mol. Biol 2005, 59, 75–84. [Google Scholar]
- Wang, Y.; Li, J. Molecular basis of plant architecture. Annu. Rev. Plant Biol 2008, 59, 253–279. [Google Scholar]
- Wang, L.; Xu, Y.; Zhang, C.; Ma, Q.; Joo, S.H.; Kim, S.K.; Xu, Z.; Chong, K. OsLIC, a novel CCCH-type zinc finger protein with transcription activation, mediates rice architecture via brassinosteroids signaling. PloS One 2008, 3, e3521. [Google Scholar]
- Zhao, S.Q.; Hu, J.; Guo, L.B.; Qian, Q.; Xue, H.W. Rice leaf inclination2, a VIN3-like protein, regulates leaf angle through modulating cell division of the collar. Cell Res 2010, 20, 935–947. [Google Scholar]
- Xu, M.; Zhu, L.; Shou, H.; Wu, P. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol 2005, 46, 1674–1681. [Google Scholar]

| Gene | Forward primer | Reverse primer |
|---|---|---|
| OsIAA4 | GCTCTTGCTGGATGGGTATGA | AGGTGATGGGCGTCTTGAAC |
| OsIAA6 | GGCTATCGTCAGCTGTCAAACA | GCAATTTGCGCATTAGTTTGG |
| OsIAA8 | CCGCTAGACGGCTACAAAGG | GGTGATGGATGCTCTGAACATG |
| OsIAA9 | CGAGAAGAAAATGGCCAATGA | ATCCCCATCACCATCCTCGTA |
| OsIAA18 | AAGAATGTGGGAAGGAGCTAACG | ATGGTGGTGAGGGACAGCAT |
| Osactin | TGGCATCTCTCAGCACATTCC | TGCACAATGGATGGGTCAGA |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, Y.; Xu, Z.-F. Ectopic Overexpression of an AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) Gene OsIAA4 in Rice Induces Morphological Changes and Reduces Responsiveness to Auxin. Int. J. Mol. Sci. 2013, 14, 13645-13656. https://doi.org/10.3390/ijms140713645
Song Y, Xu Z-F. Ectopic Overexpression of an AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) Gene OsIAA4 in Rice Induces Morphological Changes and Reduces Responsiveness to Auxin. International Journal of Molecular Sciences. 2013; 14(7):13645-13656. https://doi.org/10.3390/ijms140713645
Chicago/Turabian StyleSong, Yaling, and Zeng-Fu Xu. 2013. "Ectopic Overexpression of an AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) Gene OsIAA4 in Rice Induces Morphological Changes and Reduces Responsiveness to Auxin" International Journal of Molecular Sciences 14, no. 7: 13645-13656. https://doi.org/10.3390/ijms140713645




