Resveratrol Inhibits Ionising Irradiation-Induced Inflammation in MSCs by Activating SIRT1 and Limiting NLRP-3 Inflammasome Activation
Abstract
:1. Introduction
2. Results and Discussion
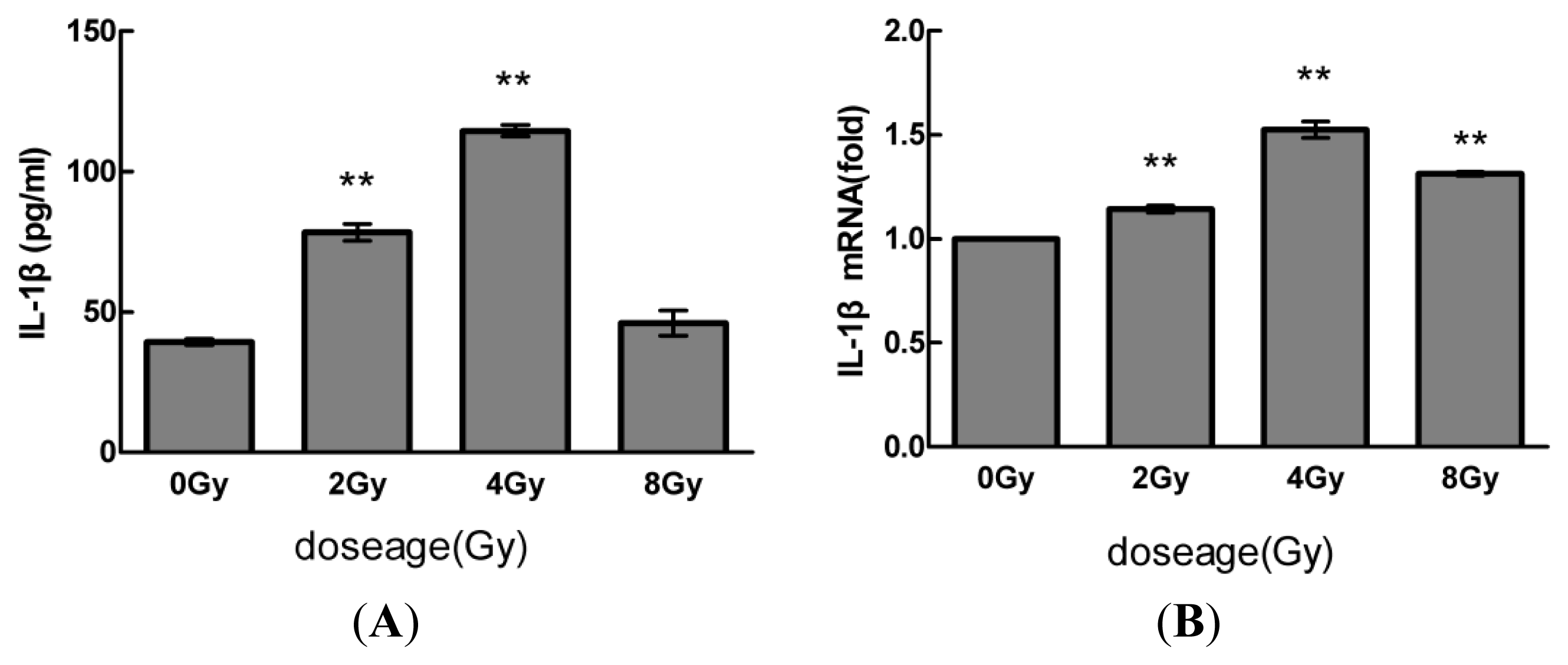
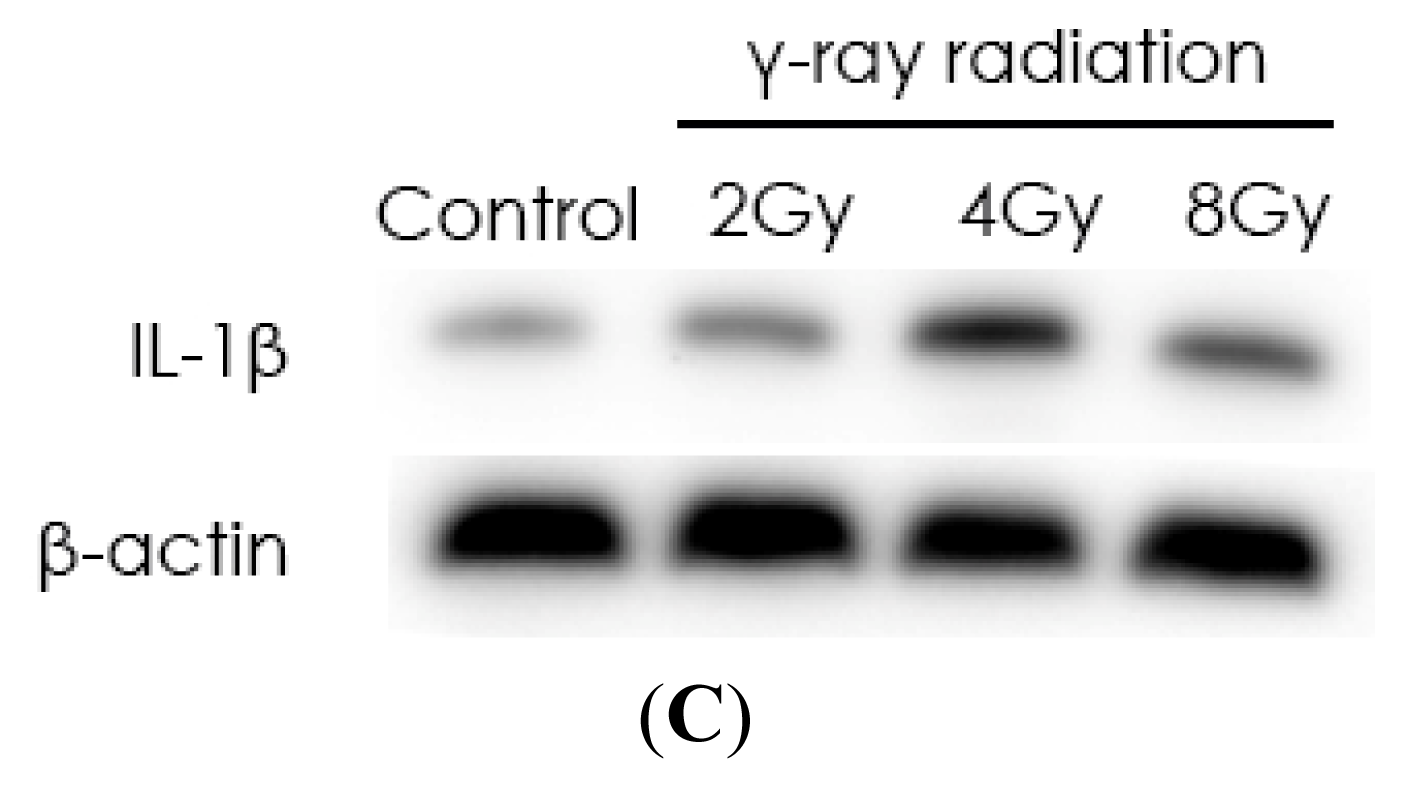
2.1. Radiation Elevates IL-1β Levels in MSCs after Radiation
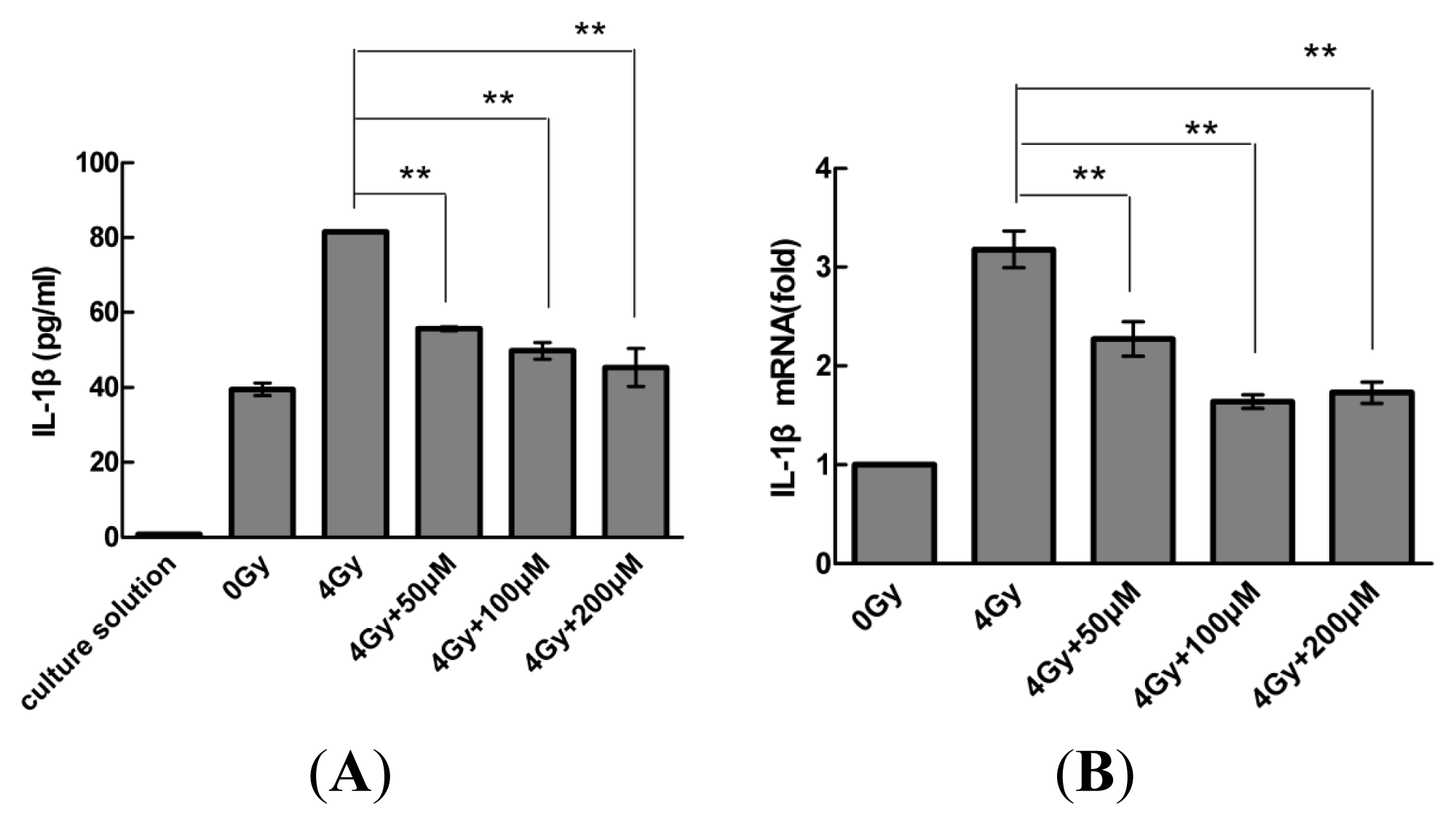
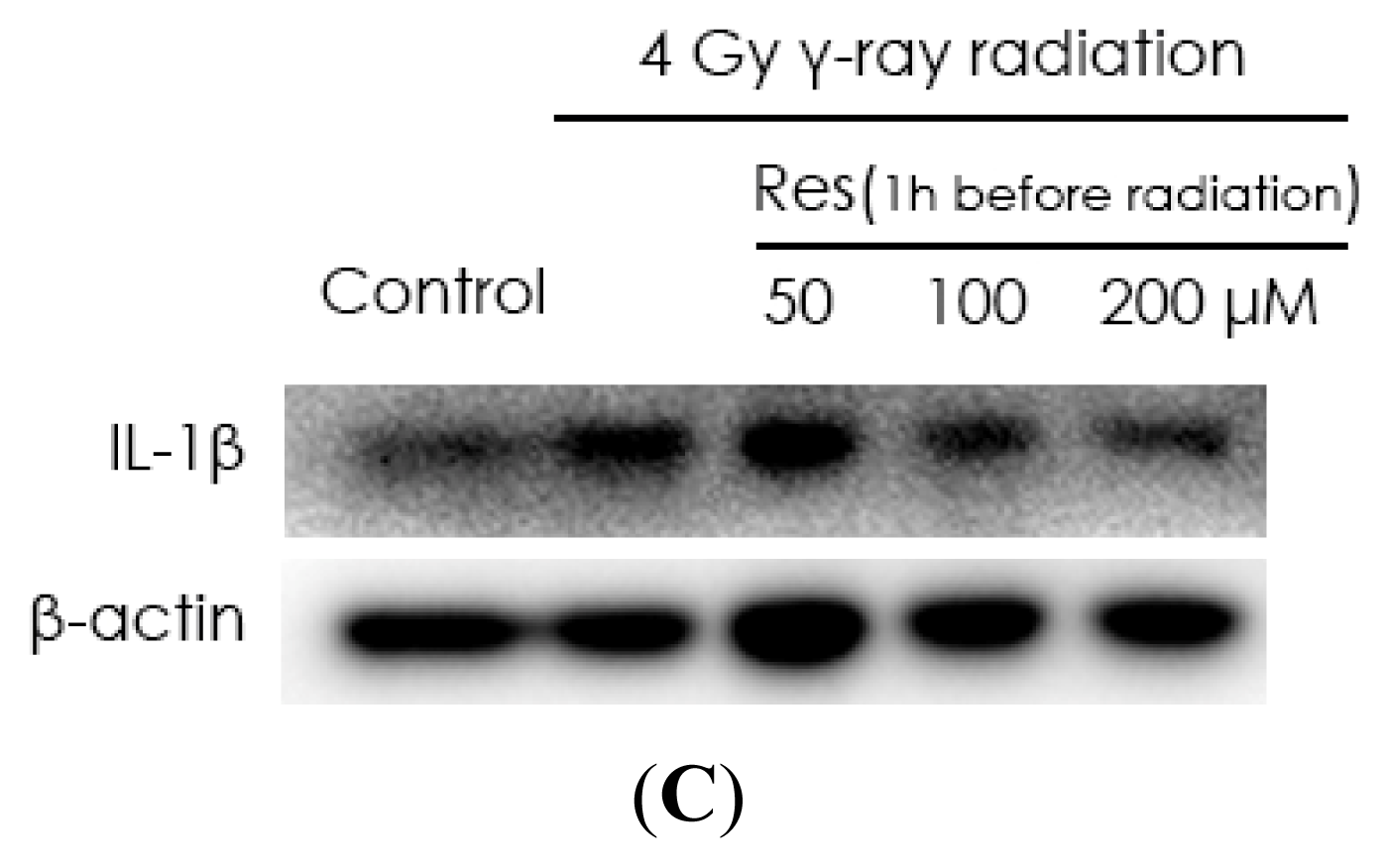
2.2. Resveratrol Reduces IL-1β Expression in MSCs
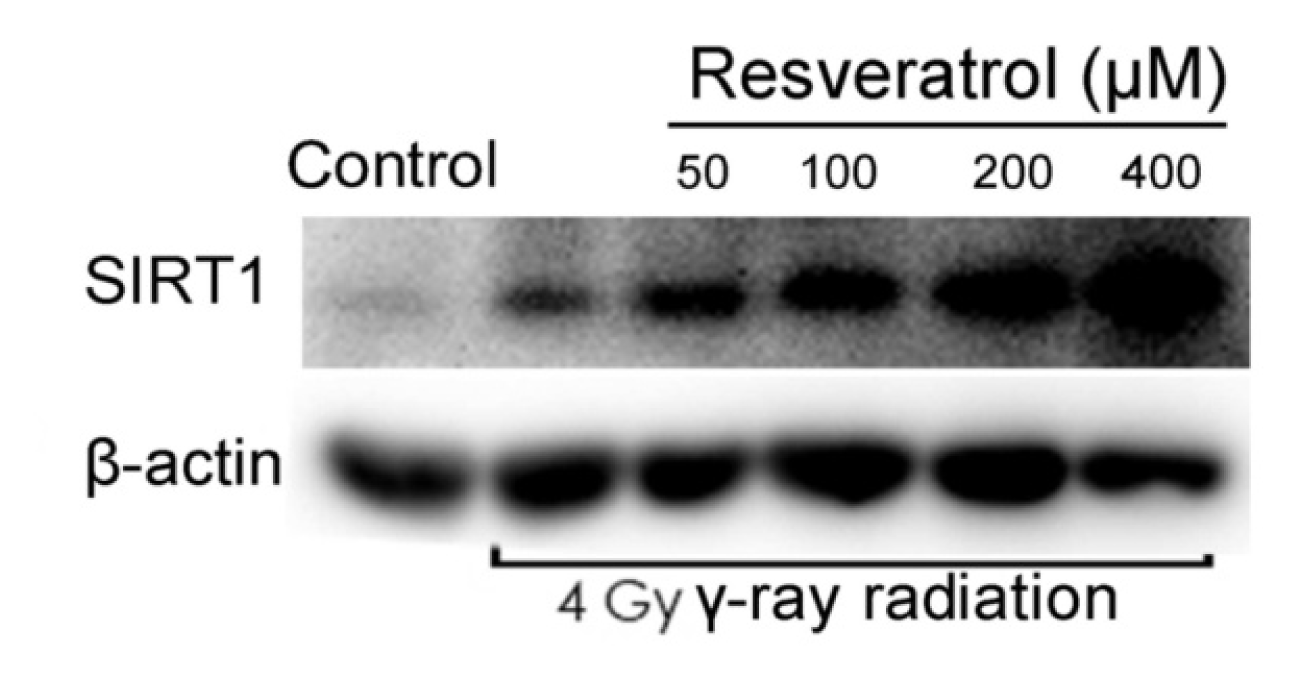
2.3. Sirt1 Is Upregulated in Resveratrol-Treated Cells
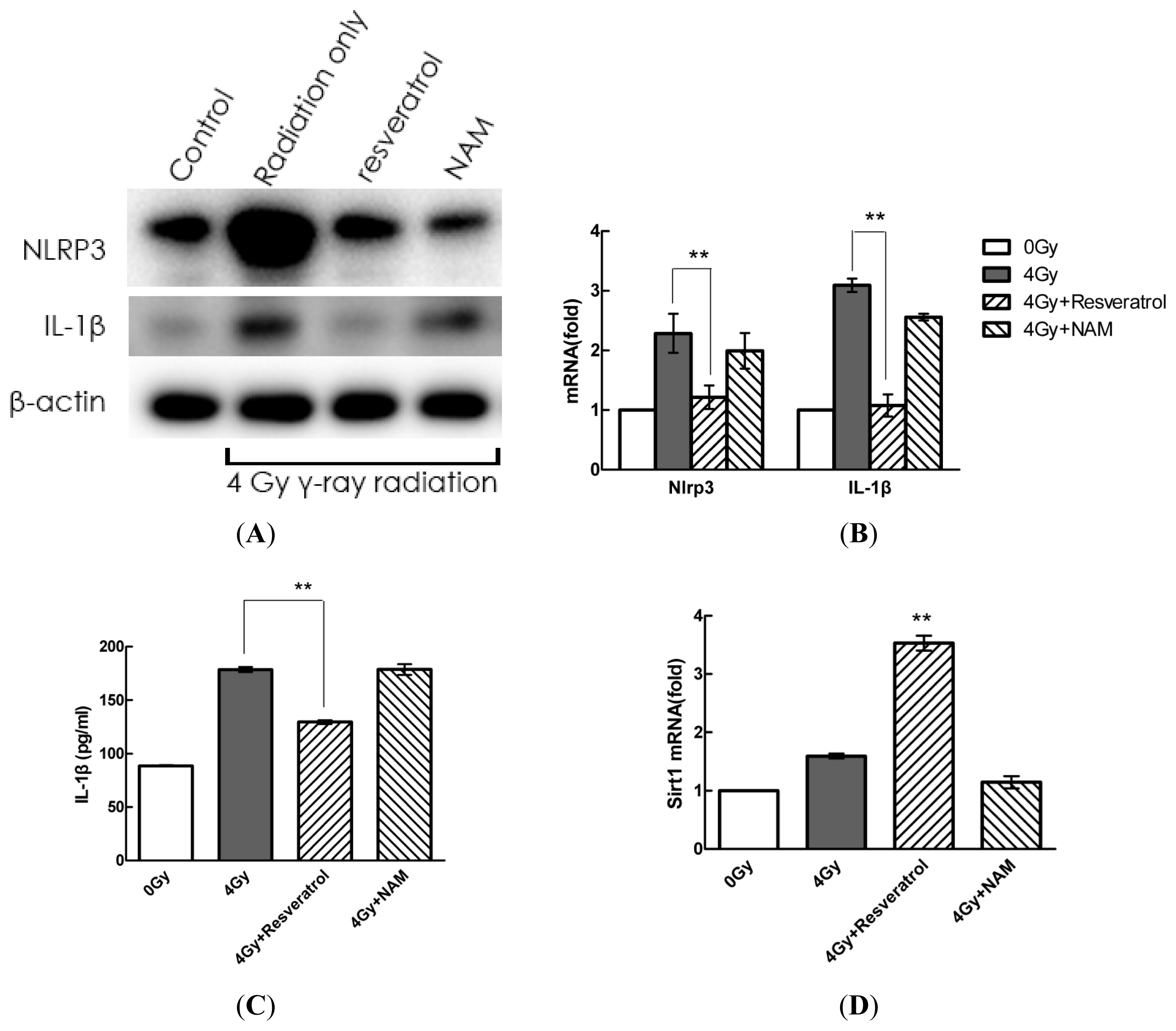
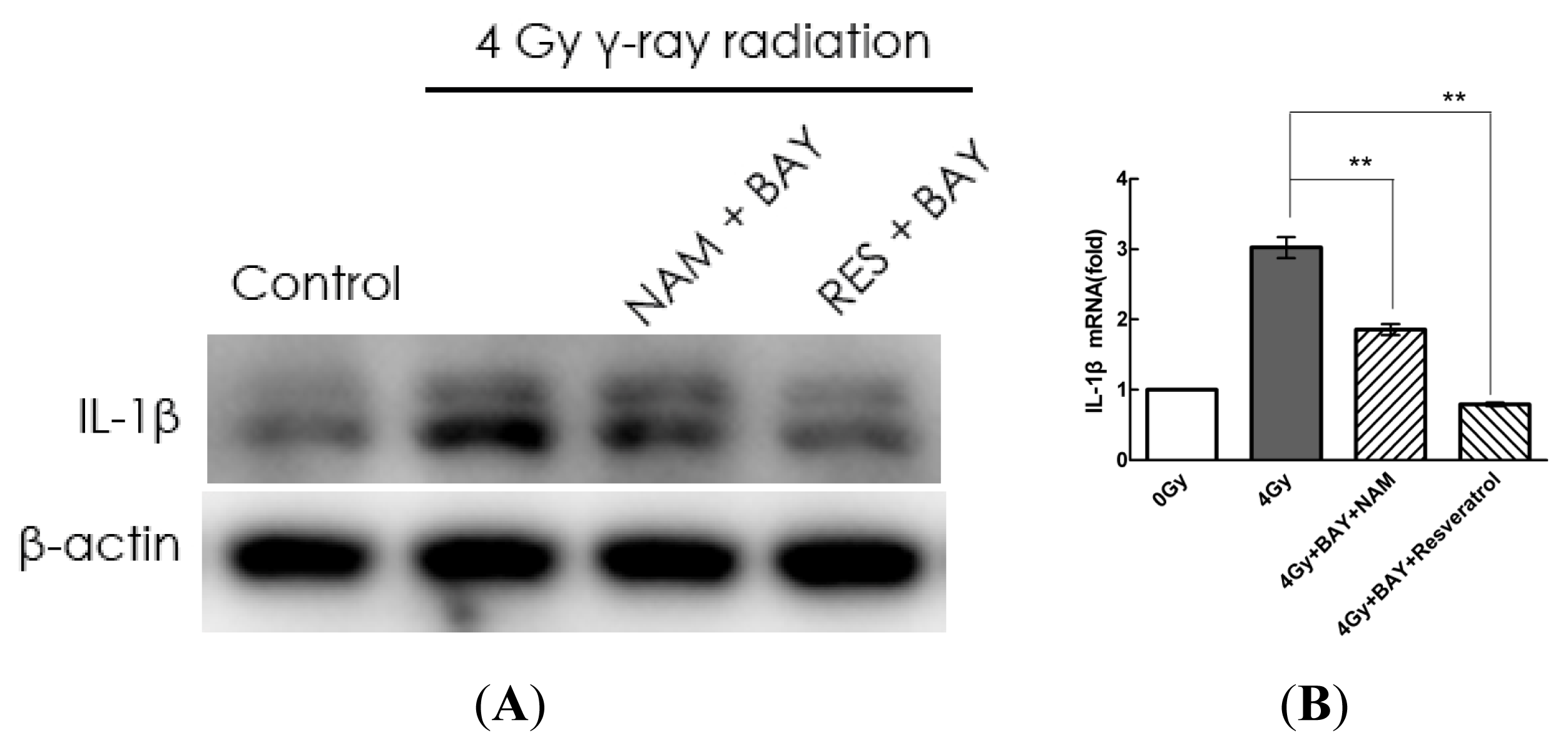
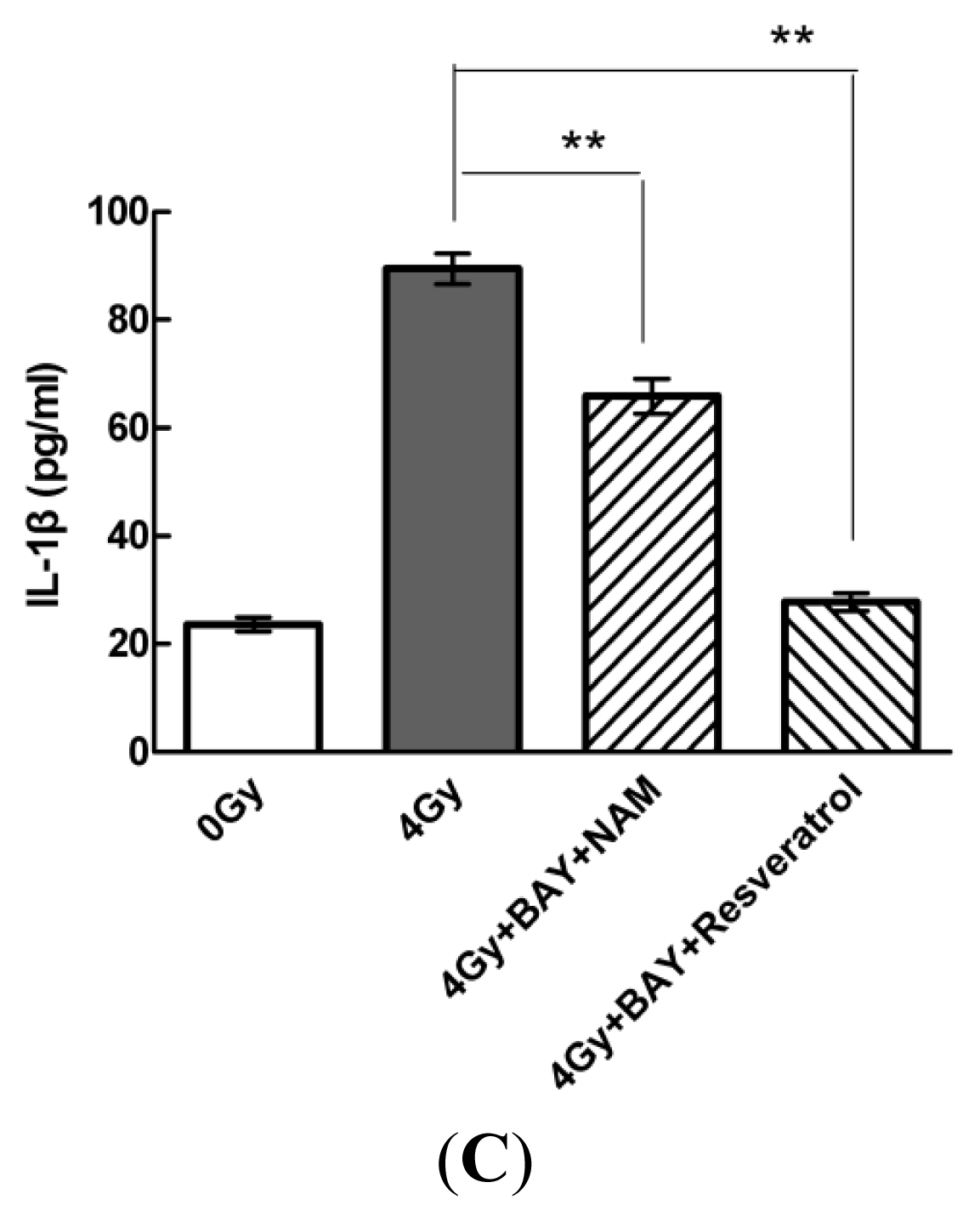
2.4. Pharmacological Modulation of Sirt1 Regulates Radiation-Induced NLRP3 and IL-1β Expression in MSCs
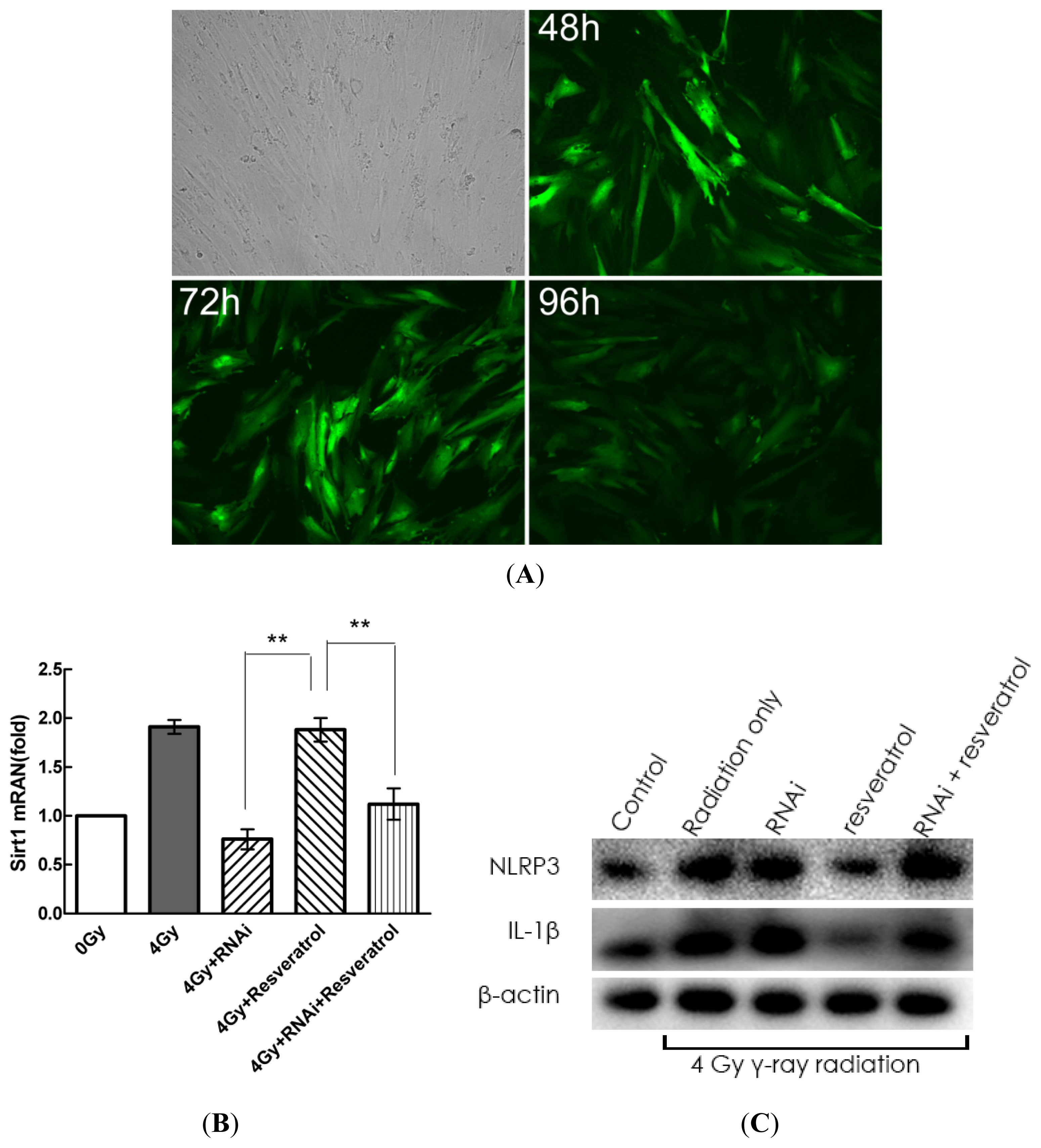
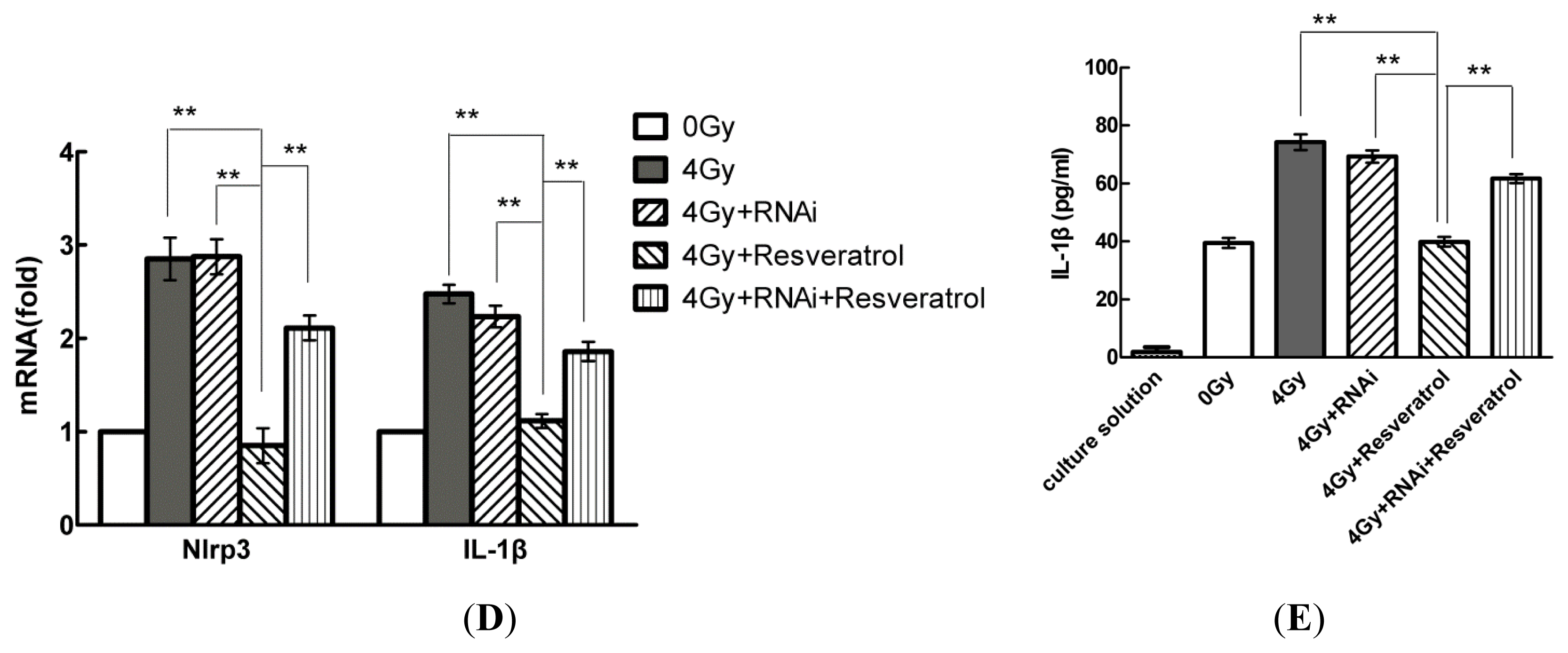
2.5. Knockdown of Sirt1 by shRNA Suppresses Resveratrol-Mediated Anti-Inflammatory Activity
2.6. Sirt1 Inhibits IL-1β Expression via the NF-κb Pathway in MSCs
3. Discussion
4. Experimental Section
4.1. Cell Lines
4.2. Reagents and Ionising Radiation
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Bernardo, M.E.; Fibbe, W.E. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann. N. Y. Acad. Sci 2012, 1266, 107–117. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar]
- Karp, J.M.; Teo, G.S.L. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar]
- Xue, J.; Li, X.; Lu, Y.; Gan, L.; Zhou, L.; Wang, Y.; Lan, J.; Liu, S.; Sun, L.; Jia, L.; et al. Gene-modified mesenchymal stem cells protect against radiation-induced lung injury. Mol. Ther 2013, 21, 456–465. [Google Scholar]
- Lim, J.-Y.; Yi, T.; Choi, J.-S.; Jang, Y.H.; Lee, S.; Kim, H.J.; Song, S.U.; Kim, Y.-M. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol 2013, 49, 136–143. [Google Scholar]
- Coppes, R.P.; Stokman, M.A. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis 2011, 17, 143–153. [Google Scholar]
- Yao, H.; Rahman, I. Perspectives on translational and therapeutic aspects of sirt1 in inflammaging and senescence. Biochem. Pharmacol 2012, 84, 1332–1339. [Google Scholar]
- Finkel, T.; Deng, C.-X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J 2007, 404, 1–13. [Google Scholar]
- Salminen, A.; Ojala, J.; Huuskonen, J.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K. Interaction of aging-associated signaling cascades: Inhibition of nf-kappab signaling by longevity factors foxos and sirt1. Cell. Mol. Life Sci 2008, 65, 1049–1058. [Google Scholar]
- Lin, Q.-Q.; Yan, C.-F.; Lin, R.; Zhang, J.-Y.; Wang, W.-R.; Yang, L.-N.; Zhang, K.-F. Sirt1 regulates tnf-α-induced expression of cd40 in 3t3-l1 adipocytes via nf-κb pathway. Cytokine 2012, 60, 447–455. [Google Scholar]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar]
- Haneklaus, M.; O’Neill, L.A.J.; Coll, R.C. Modulatory mechanisms controlling the nlrp3 inflammasome in inflammation: Recent developments. Curr. Opin. Immunol 2013, 25, 40–45. [Google Scholar]
- Dostert, C.; Pétrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar]
- Muruve, D.A.; Pétrilli, V.; Zaiss, A.K.; White, L.R.; Clark, S.A.; Ross, P.J.; Parks, R.J.; Tschopp, J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 2008, 452, 103–107. [Google Scholar]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: Nf-kappab activating pattern recognition and cytokine receptors license nlrp3 inflammasome activation by regulating nlrp3 expression. J. Immunol 2009, 183, 787–791. [Google Scholar]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol 2010, 11, 136–140. [Google Scholar]
- Nakata, R.; Takahashi, S.; Inoue, H. Recent advances in the study on resveratrol. Biol. Pharm. Bull 2012, 35, 273–279. [Google Scholar]
- Gruber, J.; Tang, S.Y.; Halliwell, B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of caenorhabditis elegans. Ann. N. Y. Acad. Sci 2007, 1100, 530–542. [Google Scholar]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the akt pathway in type 2 diabetic patients. Br. J. Nutr 2011, 106, 383–389. [Google Scholar]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev 2004, 22, 169–188. [Google Scholar]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med 2013, 54, 40–50. [Google Scholar]
- Chung, J.H.; Manganiello, V.; Dyck, J.R.B. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol 2012, 22, 546–554. [Google Scholar]
- Şimşek, G.; Gürocak, S.; Karadaǧ, N.; Karabulut, A.B.; Demirtaş, E.; Karataş, E.; Pepele, E. Protective effects of resveratrol on salivary gland damage induced by total body irradiation in rats. Laryngoscope 2012, 122, 2743–2748. [Google Scholar]
- Simsek, Y.; Gurocak, S.; Turkoz, Y.; Akpolat, N.; Celik, O.; Ozer, A.; Yılmaz, E.; Turhan, U.; Ozyalin, F. Ameliorative effects of resveratrol on acute ovarian toxicity induced by total body irradiation in young adult rats. J. Pediatr. Adolesc. Gynecol 2012, 25, 262–266. [Google Scholar]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting camp phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar]
- Yamamoto, H.; Schoonjans, K.; Auwerx, J. Sirtuin functions in health and disease. Mol. Endocrinol 2007, 21, 1745–1755. [Google Scholar]
- Zhu, X.; Liu, Q.; Wang, M.; Liang, M.; Yang, X.; Xu, X.; Zou, H.; Qiu, J. Activation of sirt1 by resveratrol inhibits tnf-α induced inflammation in fibroblasts. PLoS One 2011, 6, e27081. [Google Scholar]
- Grishman, E.K.; White, P.C.; Savani, R.C. Toll-like receptors, the nlrp3 inflammasome, and interleukin-1β in the development and progression of type 1 diabetes. Pediatr. Res 2012, 71, 626–632. [Google Scholar]
- Calzado, M.A.; Bacher, S.; Schmitz, M.L. Nf-kappab inhibitors for the treatment of inflammatory diseases and cancer. Curr. Med. Chem 2007, 14, 367–376. [Google Scholar]
- Yang, L.; Zhang, J.; Yan, C.; Zhou, J.; Lin, R.; Lin, Q.; Wang, W.; Zhang, K.; Yang, G.; Bian, X.; et al. Sirt1 regulates cd40 expression induced by tnf-α via nf-κb pathway in endothelial cells. Cell. Physiol. Biochem 2012, 30, 1287–1298. [Google Scholar]
- Lei, M.; Wang, J.-G.; Xiao, D.-M.; Fan, M.; Wang, D.-P.; Xiong, J.-Y.; Chen, Y.; Ding, Y.; Liu, S.-L. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating sirt1 and thereby suppressing nuclear factor-κb activity. Eur. J. Pharmacol 2012, 674, 73–79. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fu, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Fan, T.; Liu, J.; Su, X.; Fan, S.; Liu, Q.; et al. Resveratrol Inhibits Ionising Irradiation-Induced Inflammation in MSCs by Activating SIRT1 and Limiting NLRP-3 Inflammasome Activation. Int. J. Mol. Sci. 2013, 14, 14105-14118. https://doi.org/10.3390/ijms140714105
Fu Y, Wang Y, Du L, Xu C, Cao J, Fan T, Liu J, Su X, Fan S, Liu Q, et al. Resveratrol Inhibits Ionising Irradiation-Induced Inflammation in MSCs by Activating SIRT1 and Limiting NLRP-3 Inflammasome Activation. International Journal of Molecular Sciences. 2013; 14(7):14105-14118. https://doi.org/10.3390/ijms140714105
Chicago/Turabian StyleFu, Yue, Yan Wang, Liqing Du, Chang Xu, Jia Cao, Tiqiang Fan, Jianxiang Liu, Xu Su, Saijun Fan, Qiang Liu, and et al. 2013. "Resveratrol Inhibits Ionising Irradiation-Induced Inflammation in MSCs by Activating SIRT1 and Limiting NLRP-3 Inflammasome Activation" International Journal of Molecular Sciences 14, no. 7: 14105-14118. https://doi.org/10.3390/ijms140714105



