4.1. Numerical Details
We employ Monte Carlo (MC) simulations in the specialized isothermal-isobaric and in the grand canonical ensembles introduced briefly in Section 3. Because our intermolecular potentials are short range [see
Equations (2) and
(6)] we employ periodic boundary conditions at the planes located at
x =
±sx/2 and
y =
±sy/2 (assuming the origin of the Cartesian coordinate system to be located at the center of the simulation box). In the
z-direction, where the solid substrates are separated by a fixed distance,
sz, the implementation of hybrid anchoring [see
Equation (10)] is advantageous in avoiding spurious stresses if the ratio of 2
sz/p is non-integer, where
p denotes the pitch length characterizing chiral structures forming in that direction. This is because the anchoring function in
Equation (10a) serves to align mesogens with the
x-axis in the vicinity of the upper substrate. Because of the degenerate nature of the anchoring scenario at the lower substrate, the planar alignment of the mesogens allows a helix forming in the
z-direction to relax, even if
p/2
sz assumes non-integer values. Moreover, we create a static situation, where the helix’s orientation is fixed at one point along the
z-axis, due to directional alignment at one wall. In other words, the helix stays put along the
z-axis, which allows us to interchange summation and averaging in
Equation (20) and, therefore, facilitates the analysis of our system. In addition, the substrate causes the
z-direction to be distinct from the other two and induces a helical structure in that direction in all our simulations.
Because we are not interested in investigating any specific chiral liquid crystal, we express our quantities of interest in terms of “reduced” (i.e., dimensionless) units. For example, length is given in units of σ, energy in units of ɛff, temperature in units of ɛff/kB and pressure in units of ɛff/σ3.
For the anisotropy parameters chosen throughout this work [see Section 2,
Equation (4)] the aspect ratio of the mesogens was determined to be 1.26 [
34], taking the ratios of zeros of
ϕff (
rij,ûi,ûj) for the side-side (
ûi ·
r̂ij = 0) and end-end configurations (
|ûi ·
r̂ij| = 1) of a pair of parallel aligned mesogens (
|ûi ·
ûj| = 1) as a definition of their aspect ratio. This rather small value turns out to be advantageous, as it supports fast equilibration of the simulation sample. In combination with the simplicity of the model, it allows us to study fairly large systems containing several tens of thousands of molecules.
In the isothermal-isobaric ensemble MC simulations, we take
P|| and
T to be constant at values of 1.80 and 0.95, respectively. Under these conditions, the achiral bulk version of our model liquid crystal (
ɛ3 = 0) is sufficiently deep in the nematic phase, as reflected by the relatively high value of the global nematic order parameter,
λ ≈ 0.7. In the grand canonical MC simulations, we chose
μ = −9.588, which corresponds to
P|| ≃ 1.77 [computed via
Equation (23)], so that the thermodynamic equilibrium states considered in both ensembles are comparable.
Our results are based upon systems comprising between 5, 000 and 40, 000 mesogens and runs consisting of about 5
× 10
4 MC cycles that have been carefully equilibrated using a similar number of cycles prior to sampling any data. In the specialized isothermal-isobaric ensemble, a MC cycle consists of
N random displacements or rotations followed by one attempt to change the size of the computational cell in the
x- and
y-directions. Displacement or rotation of a mesogen are both attempted with equal probability, where the size of the displacement cube centered on a mesogen’s center-of-mass position and the angle increment of rotation are adjusted during the simulation to guarantee an overall acceptance of 40%–70% of both attempted moves. In the grand canonical simulations, an MC cycle consists of
N′ random displacements or rotations, followed by
N′ attempts to either create a new mesogen at a randomly chosen position in the system and with a randomly chosen orientation or to destroy one of the already existing ones. Both creation and destruction are also attempted with equal probability. Because the number of mesogens in the system will generally vary as a result of creation and destruction attempts,
N′ is the number of mesogens present in the simulation cell at the beginning of a new MC cycle. In our simulations, creation and destruction attempts are accepted, with a ratio of 2.2
× 10
−4. In both ensembles, we employ the standard generalized Metropolis algorithms described in Chapter 5 of [
42]. These algorithms allow one to realize numerically Markov processes that generate distributions in configuration space proportional to exp{−
β [
U(
R,Û) +
P||A −
Nβ−1 ln
A]} and exp{−
β [
U(
R,Û) −
μN] − ln
N! − 5
N ln(Λ
m/)} in the specialized isothermal-isobaric and in the grand canonical ensemble, respectively.
To save computer time and because our fluid-fluid interaction potential [see
Equation (2)] is short-range, we employ a potential cutoff of
rc = 3.0. No corrections are applied for neglected interactions beyond
rc. Moreover,
uff (
rc) remains unshifted with respect to
uff = 0. For the fluid-fluid interactions, we utilize a combination of a link-cell and a conventional Verlet neighbor list, as described in the book by Allen and Tildesley [
47] to further speed up the simulations. This latter list includes as neighbors all mesogens whose centers-of-mass are separated by a distance,
rn = 3.8, from that of a reference mesogen. By employing the two smooth walls, we make the
z-direction distinct from the other two. In order to investigate the local nematic order and the local director parallel to the wall, we divide our system along the
z-axis into 200 equally-sized slabs of volume
sxsyδz, where
δz is the thickness of each slab. For mesogens located in these slabs,
Equations (20) and
(21) are then solved as described above to obtain
λ (
r) and
n̂ (
r). During our investigations of complex chiral phases, it turned out to be practical and suitable to apply the same procedure in the
x and
y direction.
4.2. Structure of Ordered Phases
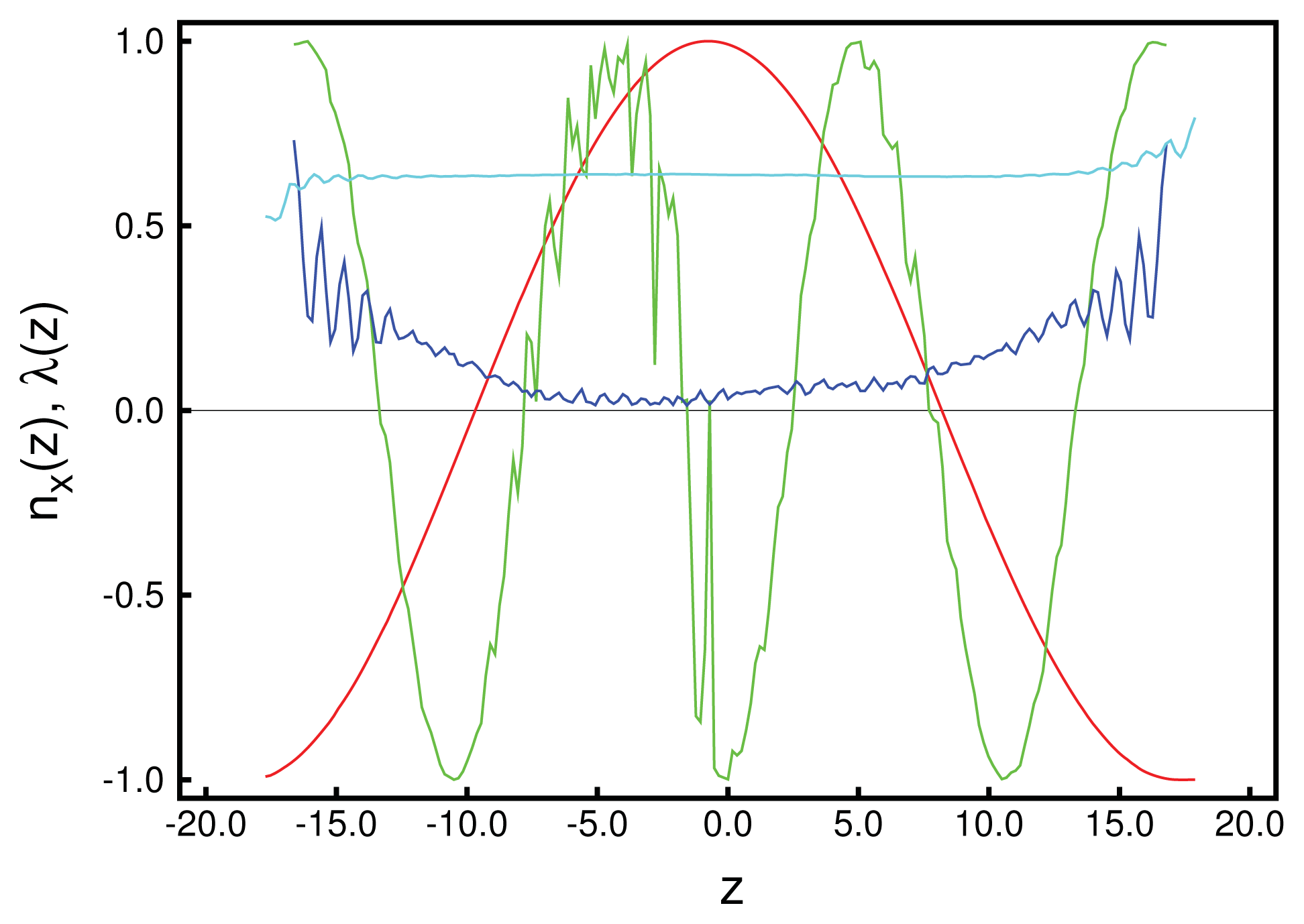
We begin the structural analysis with a discussion of the local director for a system at relatively low chirality,
ɛ3 = 0.14. Unfortunately, in general,
n̂ (
r) is a three-dimensional vector field depending on the three-dimensional vector,
r, and is, therefore, impossible to display in full. However, utilizing the fact that the
z-direction is distinct in our system, we begin by analyzing
n̂ (
z) in
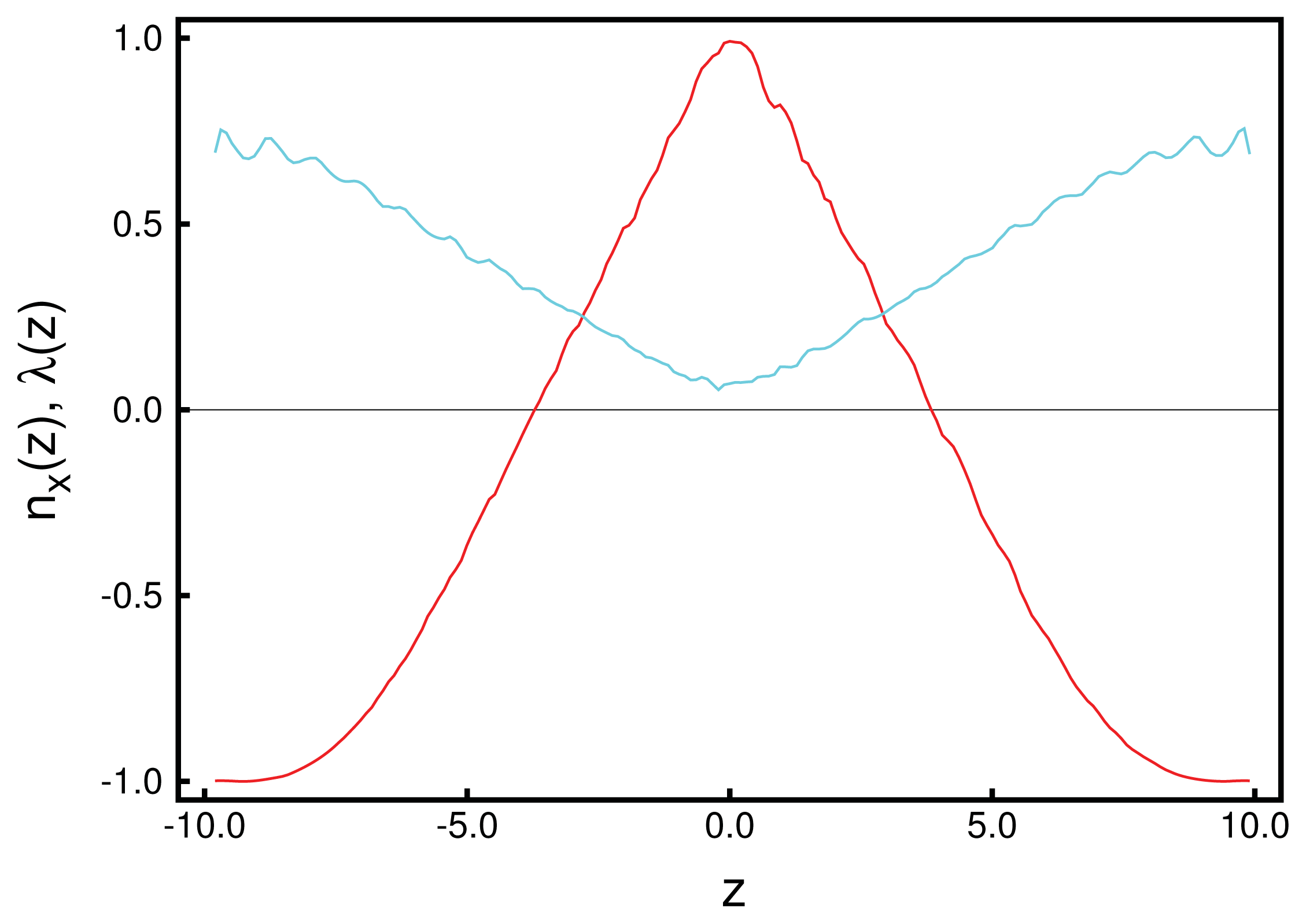
Figure 1. Data plotted in that figure illustrate the accuracy with which these curves can be obtained, provided
λ (
z) is sufficiently large. From the plot of the local order parameter,
λ (
z), it is evident that the entire confined liquid crystal exhibits a substantial nematic order in the present case. One also notices a slight asymmetry in that
λ (
z) is slightly lower at the lower substrate compared with its value at the upper substrate. This is because of the hybrid anchoring employed to generate these data [see
Equations (10)]. The monostable directional anchoring along the
x-axis causes a somewhat higher nematic order parameter compared with the degenerate planar one, because the latter is characterized by an infinite number of easy axes on the unit circle, whereas a mesogen not aligning with the
x-axis receives an energy penalty. The order parameter close to the planar-anchoring wall is even lower than in the bulk-like portion of the confined liquid crystal centered on
z = 0.
A mesogen located in that bulk-like region is surrounded by nearest neighbors in all three spatial directions. The orientation of these neighbors is tilted with respect to the reference mesogen, because at ɛ3 ≠ 0, this configuration is energetically favorable. If, on account of thermal fluctuations, the tilt angle deviates from its optimum value (at T = 0), the reference mesogen receives an energy penalty from all its neighbors. Mesogens located in the contact layer (i.e., the molecular layer in the immediate vicinity of a solid substrate) lack nearest neighbors in the direction towards the substrate. Thus, the number of nearest neighbors is reduced for these mesogens compared with those near the center of the simulation cell. As a result, the total energy penalty is smaller if the tilt angle between pairs of mesogens near the solid substrates deviates from the optimum value, which offers a possibility for somewhat larger orientational fluctuations.
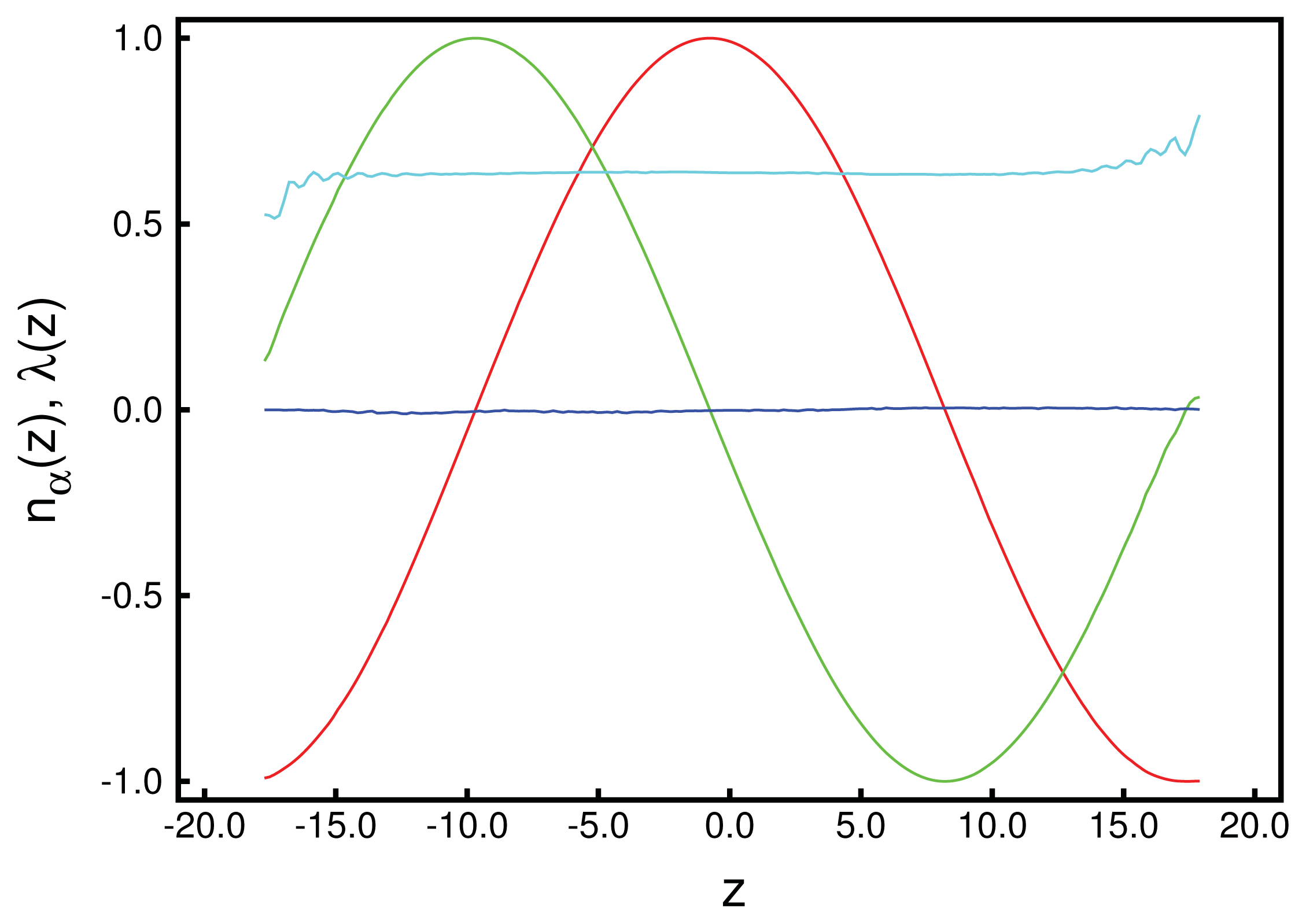
Looking next at the associated local director, n̂ (z), we realize that it varies periodically with z. Moreover, its spatial variation along the z-axis can be well described by the vector:
where
p is the pitch of the periodically varying structure. This also means that in accordance with our data in
Figure 1, the director,
n̂ (
z), rotates perpendicular to the
z-axis (
i.e., parallel to the walls), such that its
z-component vanishes. By fitting
Equation (24) to the MC data, we obtain a pitch length,
p ≃ 36.7, which exceeds the substrate separation of
sz = 35 only marginally. Hence, the periodic structure along the
z-axis does not fit
sz perfectly. Therefore, the plots of
nx (
z) and
ny (
z) in
Figure 1 are indicative of a typical cholesteric phase characterized by a single helix rotating around the
z-axis. Another characteristic feature of the cholesteric phase is that components, such as
nz (
x) and
nz (
y), of
n̂ (
r) vanish, that is, there is in-plane homogeneity of the director field along the
z-axis. This in-plane homogeneity is nicely illustrated by the plot in
Figure 2.
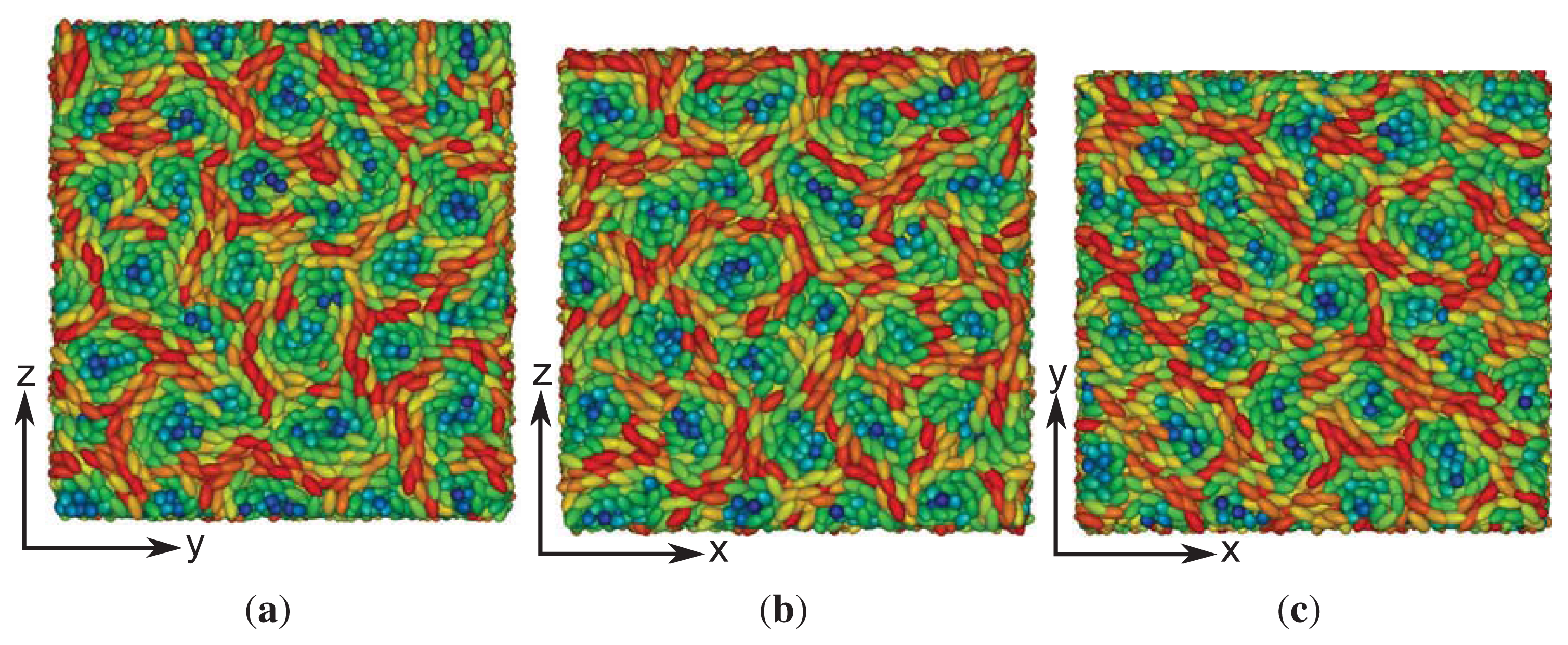
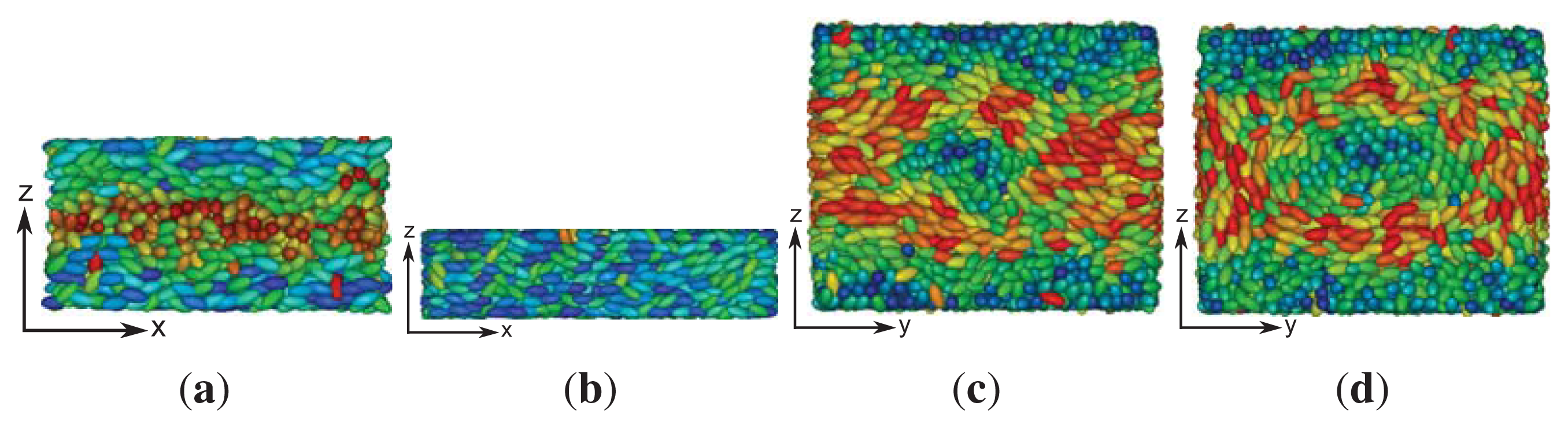
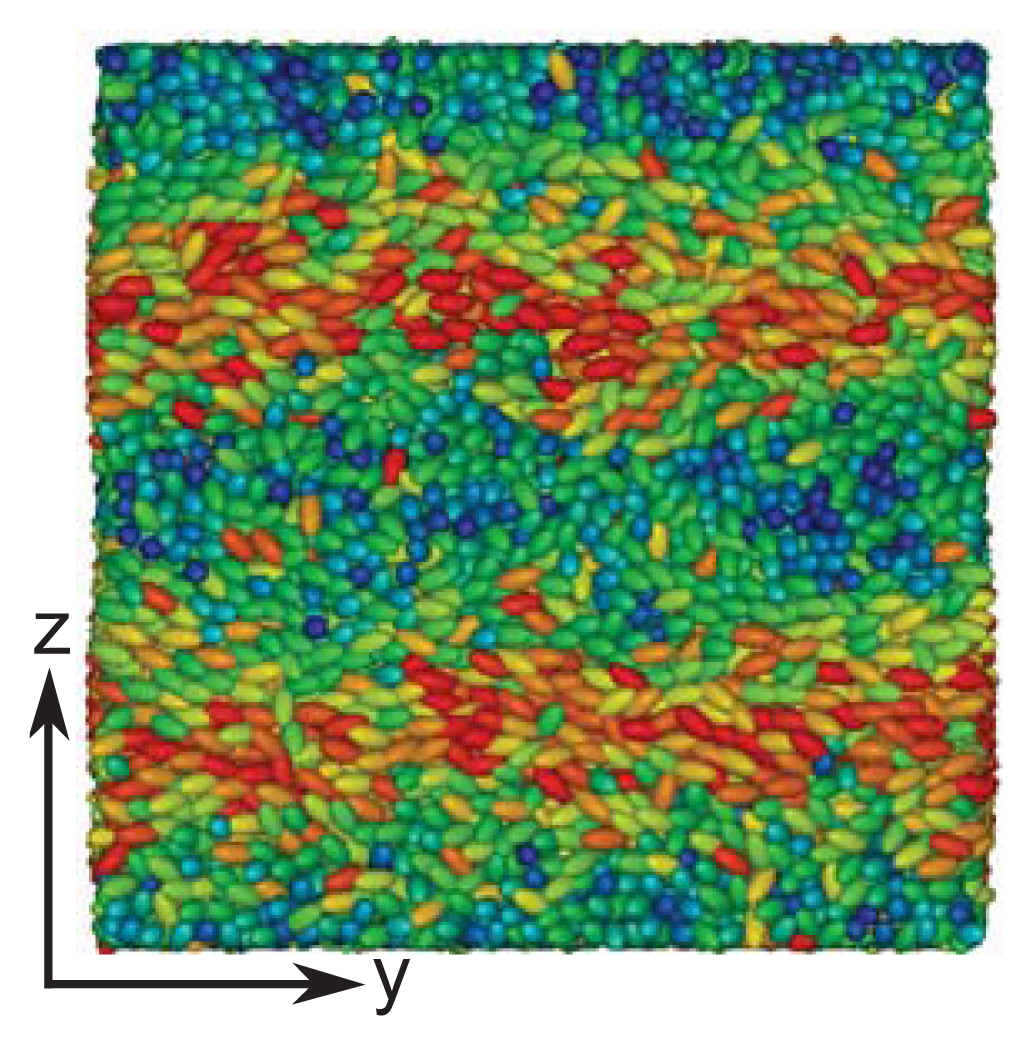
These structural features are further corroborated by the plot of a typical “snapshot” of a configuration in the cholesteric phase taken from our MC simulations (see
Figure 3). As one can see, regions can be identified that are more or less homogeneously colored. The color of these regions varies only as one moves along the
z-axis (that is, vertically), but remains nearly the same at fixed
z in the
x–
y plane. The alternating blue and red colored regions indicate that along the
z-axis, confinement by the solid substrates is such that nearly a full pitch of the helix is accommodated. That
sz = 35 is slightly too small to accommodate a full pitch can be seen from the extent of the blue colored regions at the bottom and top of the plot, which is slightly thinner for the former compared with the latter. This visual observation is fully in line with the value of
p ≃ 36.7 extracted by fitting
Equation (24) to the curves shown in
Figure 1.
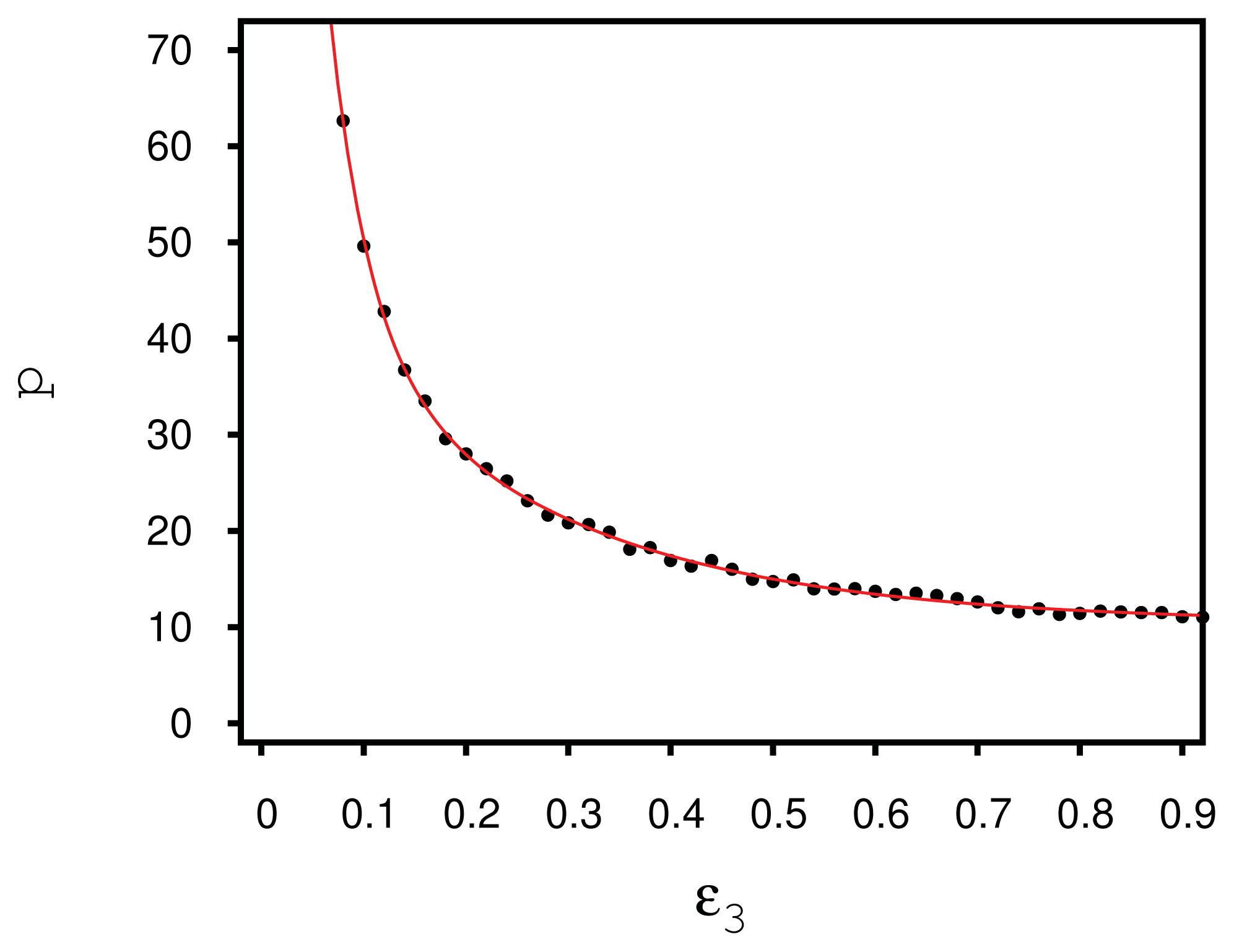
In fact, closer scrutiny reveals that the helical structures formed by the liquid crystal depend on the chirality coupling parameter,
ɛ3. Simulations in the specialized isothermal-isobaric ensemble were performed with 15,000 mesogens and a substrate distance of
sz = 26 between the hybrid aligning substrates, so that the helix forming between them is not exposed to any spurious strain in the
z-direction. The number of mesogens and the substrate distance are chosen, such that the simulation box is roughly cubic. Starting with a low value of
ɛ3 = 0.08, several simulations are performed, where the chirality is slightly increased by Δ
ɛ3 = 0.02 between subsequent simulations; in each of these simulations, the nematic director is computed as a function of
z. Employing
Equation (24), the pitch length can be determined over a wide chirality range, as demonstrated in
Figure 4. The monotonic decay of
p with increasing
ɛ3 can be attributed to the stronger twist between neighboring mesogens. For very high chiralities, we observe the pitch length to reach a plateau. For increasingly larger values of
ɛ3, highly twisted structures are energetically favored. However, the formation of these structures eventually causes even nearest neighbor molecules to deviate strongly from a side-side conformation favored energetically by the terms proportional to
ɛ1 and
ɛ2 in
Equation (4). Thus, the energetic gain in forming tilted conformations of pairs of molecules is counterbalanced by the energy penalty, due to increasingly larger deviations from these side-side arrangements.
Moreover, one notices that the local nematic order decreases with enhanced chirality. This can be seen from plots in
Figure 5, where
λ (
z) around
z = 0 and for
ɛ3 = 0.90 decays to a value characteristic of an isotropic phase. However, the liquid crystal is not isotropic locally, but does indeed form a highly structured, morphologically distinct phase under these conditions, as we shall demonstrate shortly.
As we showed above in the cholesteric phase, mesogens are aligning with a local nematic director pointing in a direction somewhere in the
x–
y plane. The orientation of the director varies only as a function of
z. This gives rise to the typical helical structure illustrated by the plots in
Figures 1,
2 and
3. However, in each of the
x–
y planes along the
z-axis, mesogens can preserve their parallel alignment, which is favored by the first two terms on the right side of
Equation (4). In other words, provided
ɛ3 is sufficiently small, nematic order can be preserved
locally.
Now, with increasing
ɛ3, in-plane, twisted conformations of mesogens become energetically more favorable, which, in turn, destroys the local, in-plane nematic order of the cholesteric phase described before. In fact, under the present conditions, the liquid crystal exhibits the complex structure reminiscent of a blue phase. This can be seen from the snapshots presented in
Figure 6, which illustrate the complex structures forming at sufficiently high values of the chirality coupling parameter,
ɛ3.
In particular, one notices that in each of the three plots, regions of blue colored mesogens at the center exist. As one moves out of these regions from the center in any radial direction, the color of the mesogens changes from blue to green and, eventually, to red, as one reaches the circumference of a region in which the mesogens are aligned with the respective line of vision. This change in color reflects a change in orientation, where mesogens at the center of each region are aligned with the line of vision, whereas along the circumference, they are oriented in an orthogonal fashion with that respective line. Hence, this orientational change is characteristic of a double-twist alignment of the mesogens. Because the global topology of the three structures depicted in
Figure 6 looks the same irrespective of the specific line of vision, we are dealing with a three-dimensional double-twist helical structure, which is characteristic of blue phases [
48].
It seems somewhat surprising that the blue phase, which is isotropic
globally, is still characterized by a small residual value of
λ (
z), as one can see from the plots in
Figure 5. However, these small values of
λ (
z) should be perceived as a consequence of the finiteness of our system, an effect which is well understood and has been analyzed quantitatively for the achiral version of our model [
37]. As before, in the cholesteric phase,
nx (
z) and
ny (
z) can be described by sine or cosine functions, indicating that there is a remaining director rotating in the
x–
y plane, but, now, with an almost vanishingly small order parameter, which also explains the relative noisiness of
nx (
z) in
Figure 5. One can also see that as one approaches the walls of the slit-pore, the plot of
nx (
z) becomes much smoother. Nevertheless, the clearly visible periodicity of
nx (
z) allows us to determine the pitch length,
p, reliably, even though the system at
ɛ3 = 0.90 is optically isotropic in a global sense, as is expected for the blue phase [
48]. Towards the substrates, nematic order increases, because of the directional anchoring that we apply at both substrate surfaces. Therefore, the present simulation of a blue phase was performed with directional anchoring at both walls, and the wall distance was estimated to give space for 3
p.
The statistical accuracy of our data can be rationalized as follows. Taking û (r) as the local orientation of a single mesogen, we can express the local nematic order parameter alternatively as:
where cos
ϑ (
r) =
û (
r) ·
n̂ (
r) is the cosine of the angle between
û (
r) and the local nematic director,
n̂ (
r),
P [
ϑ (
r)] is the distribution of orientations of a mesogen with respect to
n̂ (
r), because of the uniaxial symmetry of the mesogen, and dΩ is the solid angle. Clearly,
λ (
r) = 0 if
P [
ϑ (
r)] is uniform. If, on the other hand,
P [
ϑ (
r)] is strongly peaked, such that, for example,
P [
ϑ (
r)] =
δ [
ϑ (
r) −
π],
λ (
r) = 1, where
δ denotes the Dirac
δ-function, in other words, if the variance of the distribution,
P [
ϑ (
r)], about its mean is large, the associated local nematic order parameter,
λ (
r), will be small and
vice versa. However, a large variance in
P [
ϑ (
r)] implies a large variance of the local alignment tensor about its mean value and, therefore, a low statistical accuracy of
n̂ (
r) obtained as a solution of
Equations (20) and
(21). Plots in
Figure 5 support this line of argument.
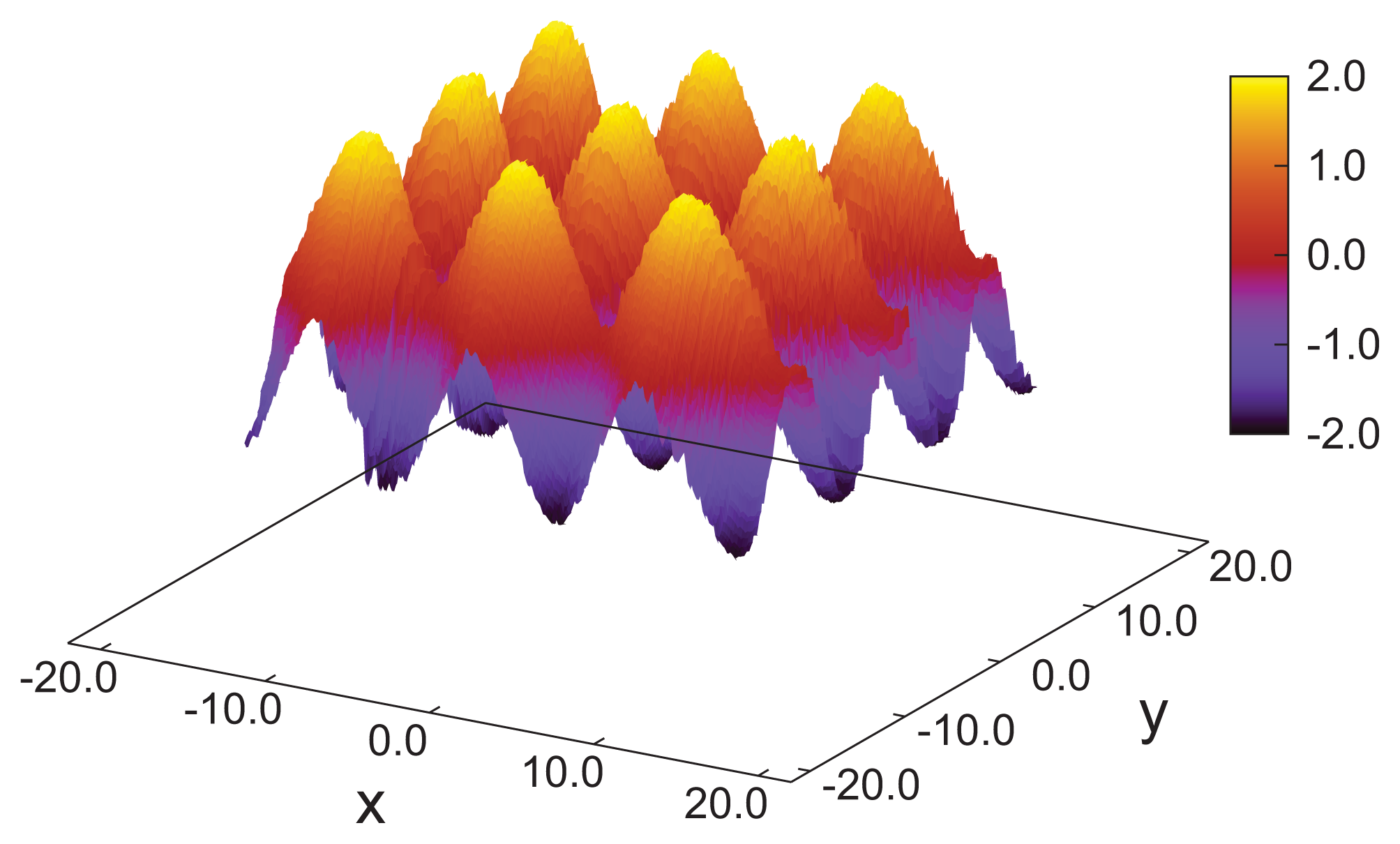
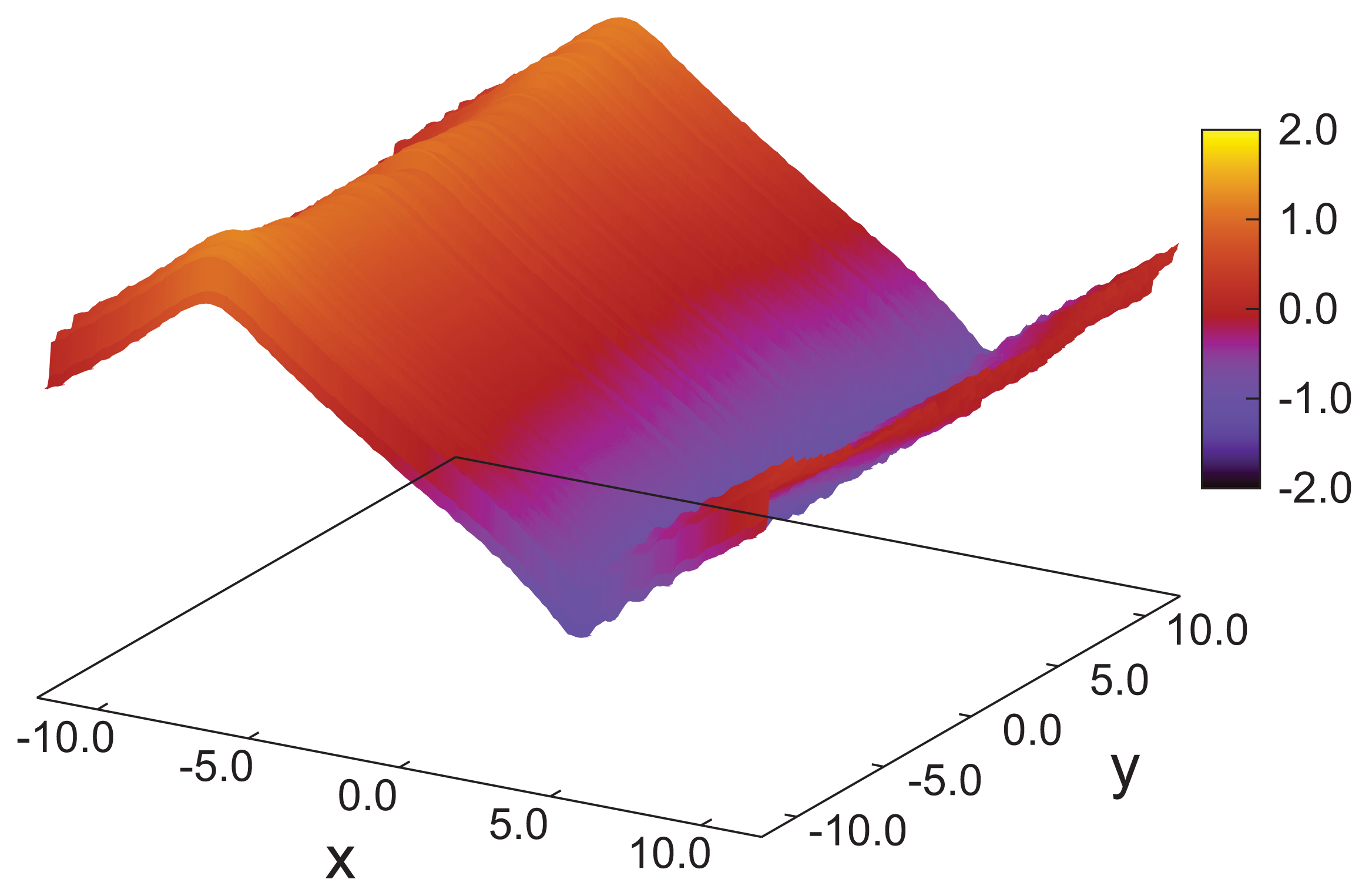
As indicated by snapshots in
Figure 6, blue phases show isotropic behavior in all three spatial directions. Based upon our results for the (components of) the director field in the
z-direction, one anticipates rotating components of the director field in the
x- and
y-directions, as well. It is particularly noteworthy that in our simulations, the helical structures forming along all three directions (
x,
y and
z) are always perpendicular to these axes. This is indeed a valid conclusion and can nicely be illustrated by plots of
nz (
x) +
nz (
y) in
Figure 7. Consider that any arbitrary point on the given surface of the plot in that figure will follow sine curves as one moves along the
x-
or y-directions, reflecting the helical structure in those directions. Together with the plot of
nx (
z) in
Figure 5, this indicates a regular double-twist helical structure in all three spatial dimensions characterized by a pitch of about ten molecular diameters. The regularity of the structure of the blue phase is consistent with the visual inspection of individual snapshots of configurations displayed in
Figure 6. Nevertheless, the particle number needs to be chosen in such a way that an integer number of half-pitches can be formed, thereby avoiding the exposition of the entire helix to spurious strains otherwise caused by applying periodic boundary conditions at the ends of the simulation cell. The optimum system size has then been determined based upon the plot presented in
Figure 4 and the ratio of 2
sz/p that we decided to employ in the simulations.
We note in passing that observing the complex three-dimensional double-twist structure of a blue phase is not only a theoretical challenge, but also an experimental one. Indeed, the typical platelet structure of the blue phases seen experimentally is a result of a non-homogeneous crystallization [
9], which is difficult to control.
Nevertheless, we stress that the results obtained in this study clearly show that MC simulations of fairly large systems—if carried out with care—are capable of reproducing three-dimensional blue-phase structures comprising a number of pitches in each direction. However, up to this point, our results do not permit us to identify the specific blue phase (I, II or III) forming in our simulations. While blue phase I and II are characterized by a regular lattice of double-twist helices, blue phase III exhibits an amorphous structure. We, therefore, speculate that the presented structure might represent a blue phase III, which also is seen experimentally for systems with higher chirality [
17]. However, the strong chirality and, therefore, the strong twist of the mesogens causes a more detailed analysis to be rather exhausting. To address this point, we amended our study by considering a system with smaller chirality,
ɛ3 = 0.30, causing a larger pitch length and containing only
N = 8
, 550 molecules. This causes a larger pitch of
p ≃ 21, such that the system can accommodate a single pitch in each spatial direction. The blue phase forming under these conditions is then identified through plots of
n̂ (
x ),
n̂ (
y) and
n̂ (
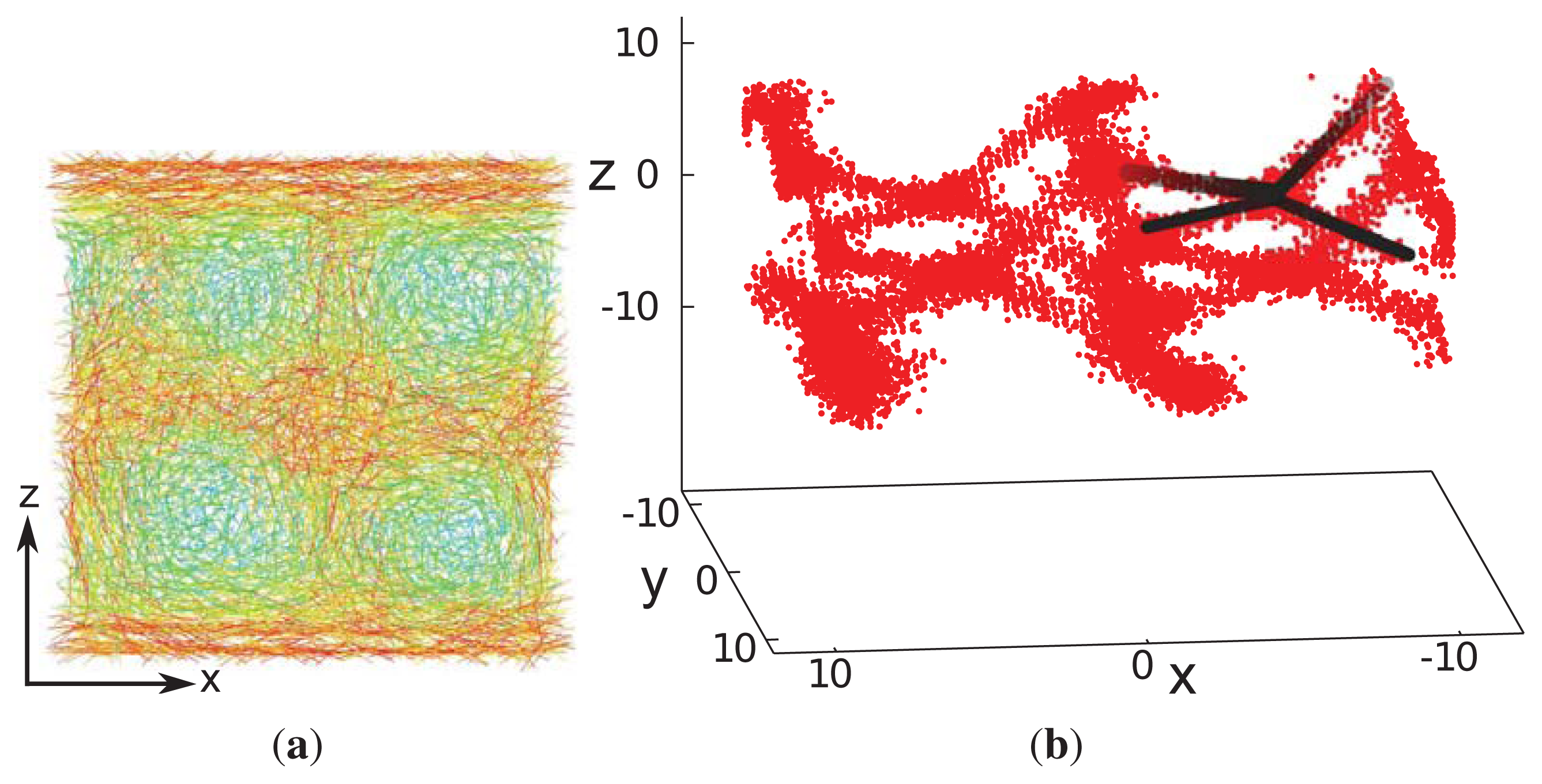
z ). Accompanying snapshots of individual configurations exhibit double-twist helices forming in parallel with the
x-,
y- and
z-axes indicating that the blue phase is not exposed to spurious strains (see
Figure 8a). In each spatial direction, the double-twist helices are arranged corresponding to a simple-cubic unit cell, which is indicative of the structure anticipated for blue phase II.
This interpretation is further corroborated by an inspection of the associated disclination lines, which we define as regions in space with a nematic order parameter,
λ (
r) ≤ 0.30. Whereas this choice of
λ (
r) is admittedly somewhat arbitrary, it can be justified by the observation that
λ ≈ 0.30 turns out to be the inflection point in plots of
λ versus the thermodynamic field driving the isotropic-nematic phase transition in the achiral analogue of our model system [
34]. As one can then see from
Figure 8b, the disclination lines form a tetrahedral structure, as expected for blue phase II [
20].
Up to this point we introduced the confining substrate merely as a technical means that allows us to determine the pitch length reliably and without interference from periodic boundary conditions. However, in parallel experiments, confining solid substrates are part of the standard setup. This prompted us to investigate the impact of the presence of these substrates on structural properties of the liquid crystal by varying
sz in small steps of magnitude Δ
sz = 0.1 between subsequent runs. In this part of our study, we employ a coupling parameter,
ɛ3 = 0.24, which is slightly bigger than the one for which the system undergoes a transformation from a cholesteric to a blue phase, according to
Figure 4. Whereas picking a certain chirality is obviously easy in our model system, one cannot easily do the same experimentally. The obvious reason is that in experimental systems, chirality is associated with a particular chemical structure and cannot be varied easily. However, the chirality of experimental systems can be altered by considering binary mixtures in which one component consists of chiral, the other one of achiral mesogens [
10].
According to the plot in
Figure 4, the pitch length is unambiguously determined by the chirality,
ɛ3. Thus, fixing the latter causes helices to form that are characterized by a fixed pitch length regardless of whether these helices are part of a cholesteric or a blue-phase structure. From the plot in
Figure 4, it is easy to determine the optimum substrate separation, such that a helix of an integer half-pitch can be accommodated, which is not strained by the mismatching substrate separation. If one now moves away from this optimum substrate separation by making
sz larger or smaller, the helix is exposed to a compressional/dilatational strain, even though the original half-integer pitch is not immediately disrupted. If the compressional/dilatational strain exceeds a certain threshold, the helix can no longer withstand the strain, and a transition to an ordinary nematic phase is observed. This is illustrated by the snapshots in
Figure 9a,b, where, starting from
sz = 12.5 (see
Figure 9a), an originally cholesteric phase is disrupted by the compressional stress associated with a reduction of the substrate separation to
sz = 8.2 (see
Figure 9b), where a conventional nematic phase is observed.
A more interesting case is observed at a substrate separation,
sz = 21.3, which is large enough, such that a double-twist helix of one full pitch can be accommodated between the confining surfaces and our choice of
ɛ3 = 0.24. At both substrates, molecules are anchored directionally [see
Equation (10a)] such that their longer axes are preferentially aligned with the
x-axis. Employing grand-canonical simulations, we are able to screen different
x–
y side lengths around one pitch length and observe, consistently, the same structure, which will be described in the following. Because our present choice of
ɛ3 = 0.24 slightly favors the formation of a blue phase and the directional anchoring would favor formation of a cholesteric phase in which the director rotates around the
z-axis, one anticipates a competition between the double-twist helix characteristic of a blue phase and a homogeneous director field in the
x–
y plane, indicative of a cholesteric phase. Indeed, an inspection of the snapshot presented in
Figure 9c illustrates the formation of both structures side by side in the same simulation.
A more quantitative analysis of this peculiar new structure is presented in
Figure 10, which shows that there is a substantial decrease of the local nematic order parameter,
λ (
z), as one approaches the center of the simulation cell at
z = 0. This is a result of the compressional/dilatational strain mentioned before. At the same time, one notices that
nx (
z) varies periodically, corresponding to a full-pitch helical structure. However, a comparison with the plots in
Figure 1 shows that for the present case, the variation of
nx (
z) cannot be described by a sine function, but exhibits a peculiar triangular shape. An even more detailed picture emerges from the plot of
nz (
x) +
nz (
y) presented in
Figure 11. Here, one notices the periodic variation of
nz (
x) +
nz (
y) along the
x-axis, reminiscent of the double-twist helix forming along that axis (see also
Figure 7) and that
nz (
x) +
nz (
y) = const along the lines of
x = const, which was a feature of cholesteric-like structures (see
Figure 2).
The mixed new structure illustrated by plots in
Figures 9,
10 and
11 is a result of a rather complex interplay between confinement, chirality and anchoring at the substrates. First, chirality slightly favors the formation of double-twist helices, according to the plot in
Figure 4. However, this requires mesogens to point towards the substrates as one moves out of the center of the double-twist helix in any radial direction. This orientation is energetically unfavorable with respect to those portions of the liquid crystal anchored at the substrates along the
x-axis. In fact, these mesogens would like to align their neighbors in a twisted, but otherwise in-plane arrangement, such that a cholesteric phase would result with a helix forming around the
z-axis. We believe that on account of thermal fluctuations, sometimes the cholesteric-like structure wins in a certain part of the system, whereas in another region and at a different stage of the evolution of our system, the double-twist helical structure may be favored.
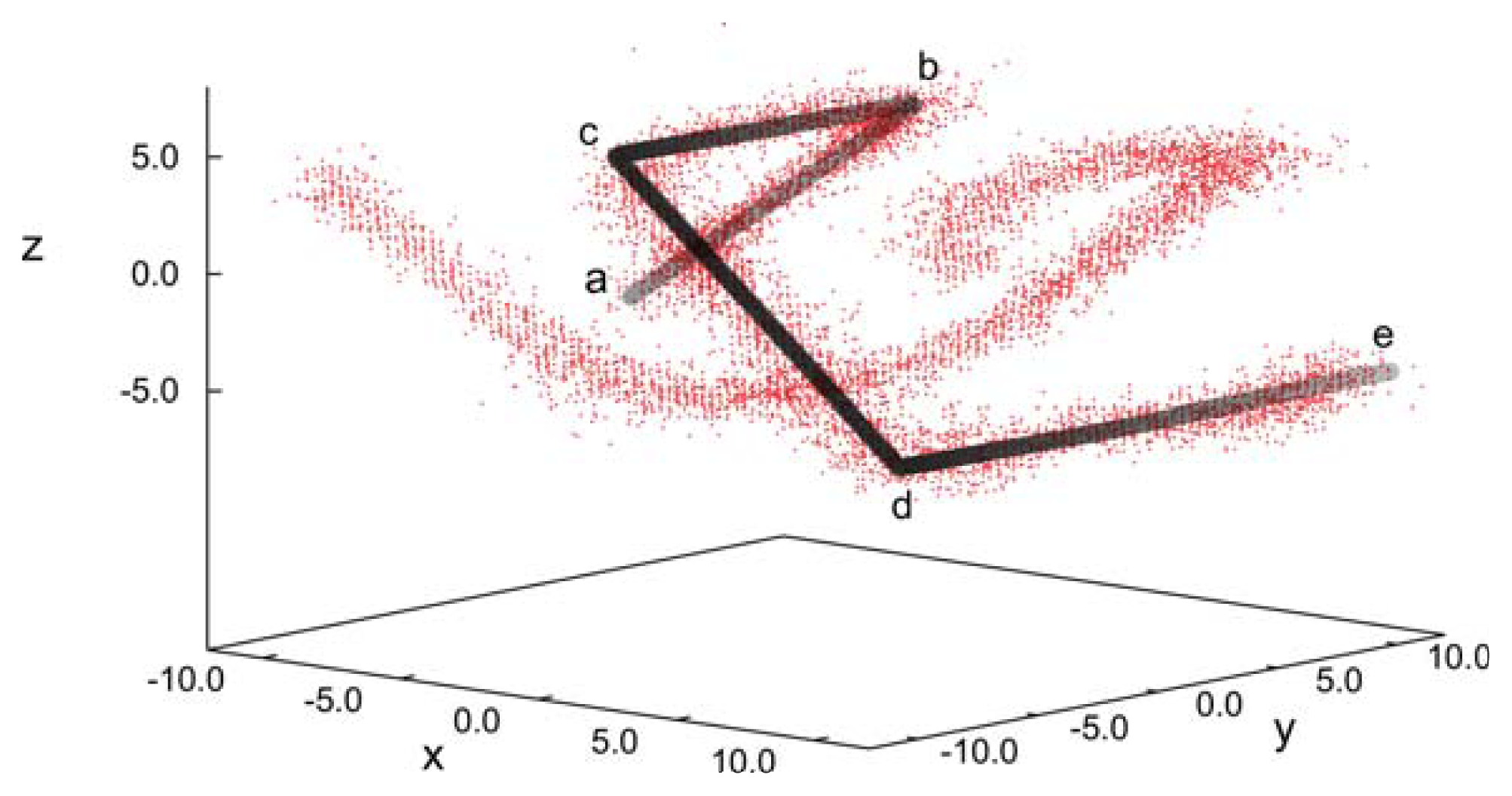
The uniqueness of the new structure observed under special confinement, chirality and anchoring conditions is also illustrated by the spatial variation of disclination lines, which we define according to the same criterion already introduced above with respect to our analysis of blue phase II. The plot of disclination lines in
Figure 12 exhibits two independent such lines that cross one another, but never join. Starting at point “a” in
Figure 12, the marked disclination line first varies along the
x-axis and, thereby, approaches the upper substrate at point “b”. It then turns rather abruptly and runs along the
y-axis in the negative direction. At point “c”, the disclination line exhibits another sharp turn into the
x-direction and descends to the plane of the lower substrate. Once that approach of the lower substrate commences at point “d” the disclination line turns again into the
y-direction and remains at constant
z, ending at point “e”, which is is equivalent to point “a”, due to periodic boundary conditions. The other disclination line plotted in
Figure 12 shows the corresponding spatial variation, as we have verified by visual inspection of the curves under different angles. This structure is distinctly different from the plot in
Figure 8b for blue phase II and, also, from the spatial variation one would expect for blue phase I [
20].
 , whose exact differential is given by:
, whose exact differential is given by: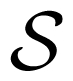 denotes entropy and μ chemical potential, such that {N, T, P||, sz}is the set of natural variables of
denotes entropy and μ chemical potential, such that {N, T, P||, sz}is the set of natural variables of
 .
. may be cast as [41]:
may be cast as [41]: is the canonical-ensemble partition function in the classical limit. As discussed in detail by Gruhn and Schoen [43] (see also [44]):
is the canonical-ensemble partition function in the classical limit. As discussed in detail by Gruhn and Schoen [43] (see also [44]): ) nx (z ), (
) nx (z ), (
 ) ny (z ), (
) ny (z ), (
 ) nz (z ). Also shown is the local nematic order parameter, λ (z ) (
) nz (z ). Also shown is the local nematic order parameter, λ (z ) (
 ).
).
 ) nx (z ), (
) nx (z ), (
 ) ny (z ), (
) ny (z ), (
 ) nz (z ). Also shown is the local nematic order parameter, λ (z ) (
) nz (z ). Also shown is the local nematic order parameter, λ (z ) (
 ).
).

 ) ɛ3 = 0.14 (cf., Figure 3) and (
) ɛ3 = 0.14 (cf., Figure 3) and (
 ) ɛ3 = 0.90. In addition, the local nematic order parameter, λ (z), is shown for ɛ3 = 0.14 (
) ɛ3 = 0.90. In addition, the local nematic order parameter, λ (z), is shown for ɛ3 = 0.14 (
 ) and ɛ3 = 0.90 (
) and ɛ3 = 0.90 (
 ). For ɛ3 = 0.90, mesogens are directionally anchored at both substrate surfaces [see Equation (10a)], because the planar alignment leads to a decrease of the order, as explained in the text and, therefore, destabilizes the structure. Here, sz is chosen to coincide roughly with 3p. For ɛ3 = 0.14, hybrid anchoring is employed [see Equations (10)], as already explained.
). For ɛ3 = 0.90, mesogens are directionally anchored at both substrate surfaces [see Equation (10a)], because the planar alignment leads to a decrease of the order, as explained in the text and, therefore, destabilizes the structure. Here, sz is chosen to coincide roughly with 3p. For ɛ3 = 0.14, hybrid anchoring is employed [see Equations (10)], as already explained.
 ) ɛ3 = 0.14 (cf., Figure 3) and (
) ɛ3 = 0.14 (cf., Figure 3) and (
 ) ɛ3 = 0.90. In addition, the local nematic order parameter, λ (z), is shown for ɛ3 = 0.14 (
) ɛ3 = 0.90. In addition, the local nematic order parameter, λ (z), is shown for ɛ3 = 0.14 (
 ) and ɛ3 = 0.90 (
) and ɛ3 = 0.90 (
 ). For ɛ3 = 0.90, mesogens are directionally anchored at both substrate surfaces [see Equation (10a)], because the planar alignment leads to a decrease of the order, as explained in the text and, therefore, destabilizes the structure. Here, sz is chosen to coincide roughly with 3p. For ɛ3 = 0.14, hybrid anchoring is employed [see Equations (10)], as already explained.
). For ɛ3 = 0.90, mesogens are directionally anchored at both substrate surfaces [see Equation (10a)], because the planar alignment leads to a decrease of the order, as explained in the text and, therefore, destabilizes the structure. Here, sz is chosen to coincide roughly with 3p. For ɛ3 = 0.14, hybrid anchoring is employed [see Equations (10)], as already explained.