Melatonin-Induced Temporal Up-Regulation of Gene Expression Related to Ubiquitin/Proteasome System (UPS) in the Human Malaria Parasite Plasmodium falciparum
Abstract
:1. Introduction
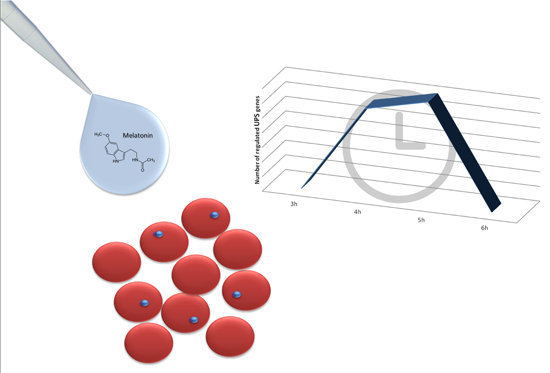
2. Results


3. Discussion
4. Experimental Section
4.1. Parasites
4.2. Real-Time PCR of UPS Genes
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar]
- Hardeland, R. Melatonin and 5-methoxytryptamine in non-metazoans. Reprod. Nutr. Dev. 1999, 39, 399–408. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Burkhardt, S.; Manchester, L.C. Melatonin in plants. Nutr. Rev. 2001, 59, 286–290. [Google Scholar]
- Roopin, M.; Yacobi, Y.Z.; Levy, O. Occurrence, diel patterns, and the influence of melatonin on the photosynthetic performance of cultured Symbiodinium. J. Pineal Res. 2013, 55, 89–100. [Google Scholar]
- Park, S.; Byeon, Y.; Kim, Y.S.; Back, K. Kinetic analysis of purified recombinant rice N-acetylserotonin methyltransferase and peak melatonin production in etiolated rice shoots. J. Pineal Res. 2013, 54, 139–144. [Google Scholar]
- Bubenik, G.A. Localization of melatonin in the digestive tract of the rat. Effect of maturation, diurnal variation, melatonin treatment and pinealectomy. Horm. Res. 1980, 12, 313–323. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Zhang, M.; Weintraub, S.T.; Cabrera, J.; Sainz, R.M.; Mayo, J.C. Identification of highly elevated levels of melatonin in bone marrow: Its origin and significance. Biochim. Biophys. Acta Gen. Subj. 1999, 1472, 206–214. [Google Scholar]
- Guerrero, J.M.; Reiter, R.J. Melatonin-immune system relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; Garc#xED;a-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar]
- Garcia, C.R.; Markus, R.P.; Madeira, L. Tertian and quartan fevers: Temporal regulation in malarial infection. J. Biol. Rhythm. 2001, 16, 436–443. [Google Scholar]
- Hotta, C.T.; Gazarini, M.L.; Beraldo, F.H.; Varotti, F.P.; Lopes, C.; Markus, R.P.; Pozzan, T.; Garcia, C.R. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat. Cell Biol. 2000, 2, 466–468. [Google Scholar]
- Macías, M.; Rodríguez-Cabezas, M.N.; Reiter, R.J.; Osuna, A.; Acuna-Castroviejo, D. Presence and effects of melatonin in Trypanosoma cruzi. J. Pineal Res. 1999, 27, 86–94. [Google Scholar]
- Bagnaresi, P.; Nakabashi, M.; Thomas, A.P.; Reiter, R.J.; Garcia, C.R.S. The role of melatonin in parasite biology. Mol. Biochem. Parasitol. 2012, 181, 1–6. [Google Scholar]
- Beraldo, F.H.; Almeida, F.M.; da Silva, A.M.; Garcia, C.R.S. Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. J. Cell Biol. 2005, 170, 551–557. [Google Scholar]
- Koyama, F.C.; Ribeiro, R.Y.; Garcia, J.L.; Azevedo, M.F.; Chakrabarti, D.; Garcia, C.R.S. Ubiquitin proteasome system and the atypical kinase PfPK7 are involved in melatonin signaling in Plasmodium falciparum. J. Pineal Res. 2012, 53, 147–153. [Google Scholar]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar]
- Ciechanover, A. The ubiquitin—Proteasome pathway: On protein death and cell life. EMBO J. 1998, 17, 7151–7160. [Google Scholar]
- Hunt, P.; Afonso, A.; Creasey, A.; Culleton, R.; Sidhu, A.B.S.; Logan, J.; Valderramos, S.G.; McNae, I.; Cheesman, S.; do Rosario, V.; et al. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol. Microbiol. 2007, 65, 27–40. [Google Scholar]
- Sung, J.H.; Cho, E.H.; Kim, M.O.; Koh, P.O. Identification of proteins differentially expressed by melatonin treatment in cerebral ischemic injury—A proteomics approach. J. Pineal Res. 2009, 46, 300–306. [Google Scholar]
- Wang, P.; Sun, X.; Chang, C.; Feng, F.; Liang, D.; Cheng, L.; Ma, F. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 2013, 55, 424–434. [Google Scholar]
- Lindenthal, C.; Weich, N.; Chia, Y.S.; Heussler, V.; Klinkert, M.Q. The proteasome inhibitor MLN-273 blocks exoerythrocytic and erythrocytic development of Plasmodium parasites. Parasitology 2005, 131, 37–44. [Google Scholar]
- Reynolds, J.M.; el Bissati, K.; Brandenburg, J.; Günzl, A.; Mamoun, C.B. Antimalarial activity of the anticancer and proteasome inhibitor bortezomib and its analog ZL3B. BMC Clin. Pharmacol. 2007, 7, 13. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Hideshima, T.; Richardson, P.G.; Russo, D.; Ribatti, D.; Vacca, A.; Dammacco, F.; Anderson, K.C. Bortezomib as an antitumor agent. Curr. Pharm. Biotechnol. 2006, 7, 441–448. [Google Scholar]
- Ponts, N.; Saraf, A.; Chung, D.-W.D.; Harris, A.; Prudhomme, J.; Washburn, M.P.; Florens, L.; le Roch, K.G. Unraveling the ubiquitome of the human malaria parasite. J. Biol. Chem. 2011, 286, 40320–40330. [Google Scholar]
- Bozdech, Z.; Llinas, M.; Pulliam, B.L.; Wong, E.D.; Zhu, J.; DeRisi, J.L. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003, 1. [Google Scholar] [CrossRef] [Green Version]
- Horrocks, P.; Newbold, C.I. Intraerythrocytic polyubiquitin expression in Plasmodium falciparum is subjected to developmental and heat-hock control. Mol. Biochem. Parasitol. 2000, 105, 115–125. [Google Scholar]
- Woelk, T.; Sigismund, S.; Penengo, L.; Polo, S. The ubiquitination code: A signalling problem. Cell Div. 2007, 2. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar]
- Maier, A.G.; Cooke, B.M.; Cowman, A.F.; Tilley, L. Malaria parasite proteins that remodel the host erythrocyte. Nat. Rev. Microbiol. 2009, 7, 341–354. [Google Scholar]
- Garcia, C.R.; Takeuschi, M.; Yoshioka, K.; Miyamoto, H. Imaging Plasmodium falciparum-infected ghost and parasite by atomic force microscopy. J. Struct. Biol. 1997, 119, 92–98. [Google Scholar]
- Singh, S.; Alam, M.M.; Pal-Bhowmick, I.; Brzostowski, J.A.; Chitnis, C.E. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010, 6, e1000746. [Google Scholar]
- Beraldo, F.H.; Garcia, C.R.S. Products of tryptophan catabolism induce Ca2+ release and modulate the cell cycle of Plasmodium falciparum malaria parasites. J. Pineal Res. 2005, 39, 224–230. [Google Scholar]
- Gazarini, M.L.; Beraldo, F.H.; Almeida, F.M.; Bootman, M.; da Silva, A.M.; Garcia, C.R.S. Melatonin triggers PKA activation in the rodent malaria parasite Plasmodium chabaudi. J. Pineal Res. 2011, 50, 64–70. [Google Scholar]
- Lima, W.R.; Holder, A.A.; Garcia, C.R.S. Melatonin signaling and its modulation of PfNF-YB transcription factor expression in Plasmodium falciparum. Int. J. Mol. Sci. 2013, 14, 13704–13718. [Google Scholar]
- Passos, A.P.; Garcia, C.R. Characterization of Ca2+ transport activity associated with a non-mitochondrial calcium pool in the rodent malaria parasite P. chabaudi. Biochem. Mol. Biol. Int. 1997, 42, 919–925. [Google Scholar]
- Hotta, C.T.; Markus, R.P.; Garcia, C.R.S. Melatonin and N-acetyl-serotonin cross the red blood cell membrane and evoke calcium mobilization in malarial parasites. Braz. J. Med. Biol. Res. 2003, 36, 1583–1587. [Google Scholar]
- Beraldo, F.H.; Mikoshiba, K.; Garcia, C.R.S. Human malarial parasite, Plasmodium falciparum, displays capacitative calcium entry: 2-Aminoethyl diphenylborinate blocks the signal transduction pathway of melatonin action on the P. falciparum cell cycle. J. Pineal Res. 2007, 43, 360–364. [Google Scholar]
- Madeira, L.; Galante, P.A.F.; Budu, A.; Azevedo, M.F.; Malnic, B.; Garcia, C.R.S. Genome-wide detection of serpentine receptor-like proteins in malaria parasites. PLoS One 2008, 3. [Google Scholar] [CrossRef]
- Farias, S.L.; Gazarini, M.L.; Melo, R.L.; Hirata, I.Y.; Juliano, M.A.; Juliano, L.; Garcia, C.R.S. Cysteine-protease activity elicited by Ca2+ stimulus in Plasmodium. Mol. Biochem. Parasitol. 2005, 141, 71–79. [Google Scholar]
- Vriend, J.; Reiter, R.J. Melatonin and ubiquitin: What’s the connection? Cell. Mol. Life Sci. 2014, 71, 3409–3418. [Google Scholar]
- Koyama, F.C.; Carvalho, T.L.G.; Alves, E.; da Silva, H.B.; de Azevedo, M.F.; Hemerly, A.S.; Garcia, C.R.S. The structurally related auxin and melatonin tryptophan-derivatives and their roles in Arabidopsis thaliana and in the human malaria parasite Plasmodium falciparum. J. Eukaryot. Microbiol. 2013, 60, 646–651. [Google Scholar]
- Muralidharan, V.; Oksman, A.; Iwamoto, M.; Wandless, T.J.; Goldberg, D.E. Asparagine repeat function in a Plasmodium falciparum protein assessed via a regulatable fluorescent affinity tag. Proc. Natl. Acad. Sci. USA 2011, 108, 4411–4416. [Google Scholar]
- Li, Z.; Wang, C.C. Functional characterization of the 11 non-ATPase subunit proteins in the trypanosome 19 S proteasomal regulatory complex. J. Biol. Chem. 2002, 277, 42686–42693. [Google Scholar]
- De Diego, J.L.; Katz, J.M.; Marshall, P.; Manning, B.; Gutierrez, J.E.; Nussenzweig, V.; Gonzalez, J. The ubiquitin-proteasome pathway plays an essential role in proteolysis during Trypanosoma cruzi remodeling. Biochemistry 2001, 40, 1053–1062. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyama, F.C.; Azevedo, M.F.; Budu, A.; Chakrabarti, D.; Garcia, C.R.S. Melatonin-Induced Temporal Up-Regulation of Gene Expression Related to Ubiquitin/Proteasome System (UPS) in the Human Malaria Parasite Plasmodium falciparum. Int. J. Mol. Sci. 2014, 15, 22320-22330. https://doi.org/10.3390/ijms151222320
Koyama FC, Azevedo MF, Budu A, Chakrabarti D, Garcia CRS. Melatonin-Induced Temporal Up-Regulation of Gene Expression Related to Ubiquitin/Proteasome System (UPS) in the Human Malaria Parasite Plasmodium falciparum. International Journal of Molecular Sciences. 2014; 15(12):22320-22330. https://doi.org/10.3390/ijms151222320
Chicago/Turabian StyleKoyama, Fernanda C., Mauro F. Azevedo, Alexandre Budu, Debopam Chakrabarti, and Célia R. S. Garcia. 2014. "Melatonin-Induced Temporal Up-Regulation of Gene Expression Related to Ubiquitin/Proteasome System (UPS) in the Human Malaria Parasite Plasmodium falciparum" International Journal of Molecular Sciences 15, no. 12: 22320-22330. https://doi.org/10.3390/ijms151222320