Topical N-Acetylcysteine Accelerates Wound Healing in Vitro and in Vivo via the PKC/Stat3 Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. N-Acetylcysteine (Nac) Enhanced Proliferation of CCD-966SK Cells
2.1.2. Nac Increased the glutathione (GSH) Level of the CCD-966SK Cells
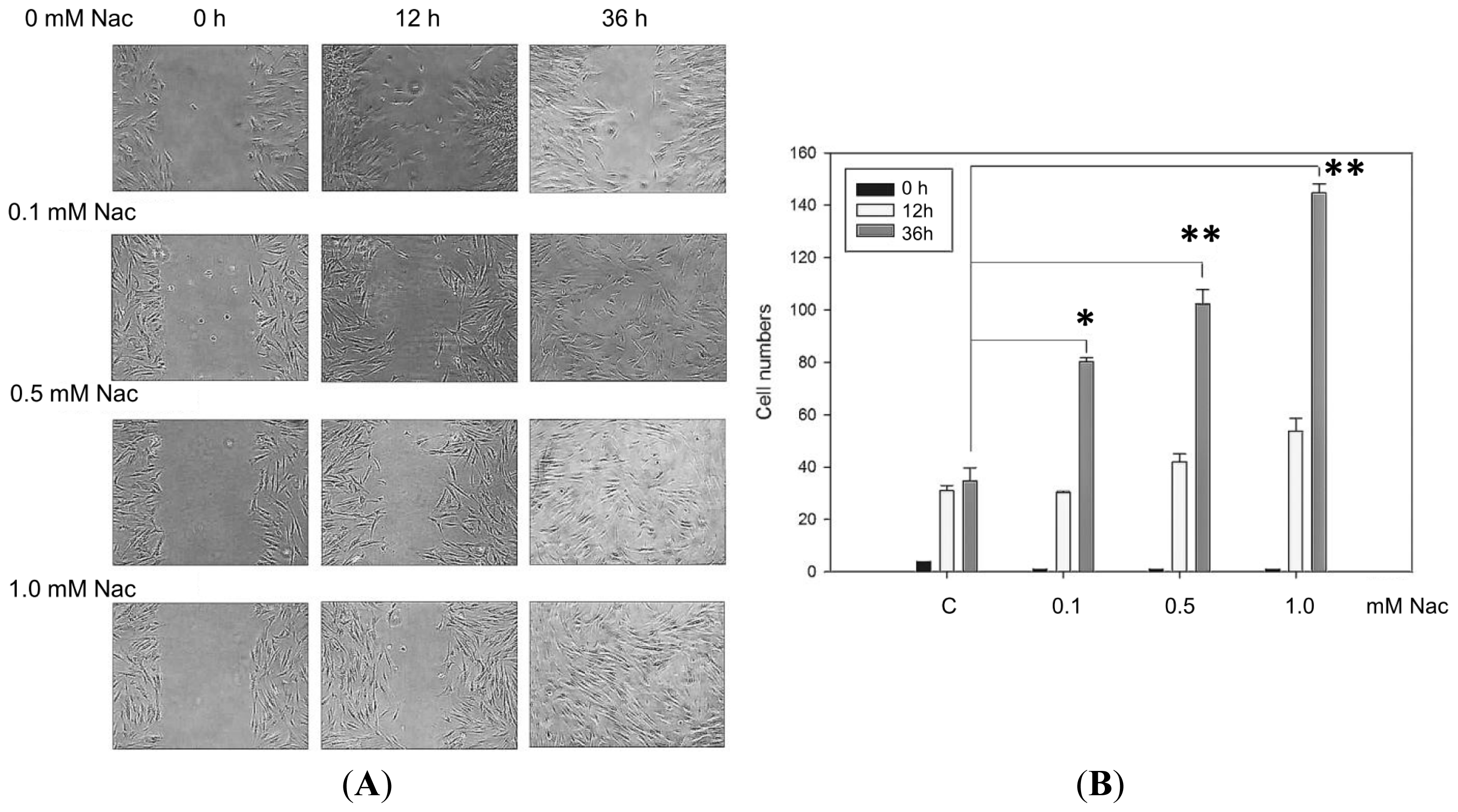
2.1.3. Wound Healing Ability of the CCD-966SK Cells Treated with Nac
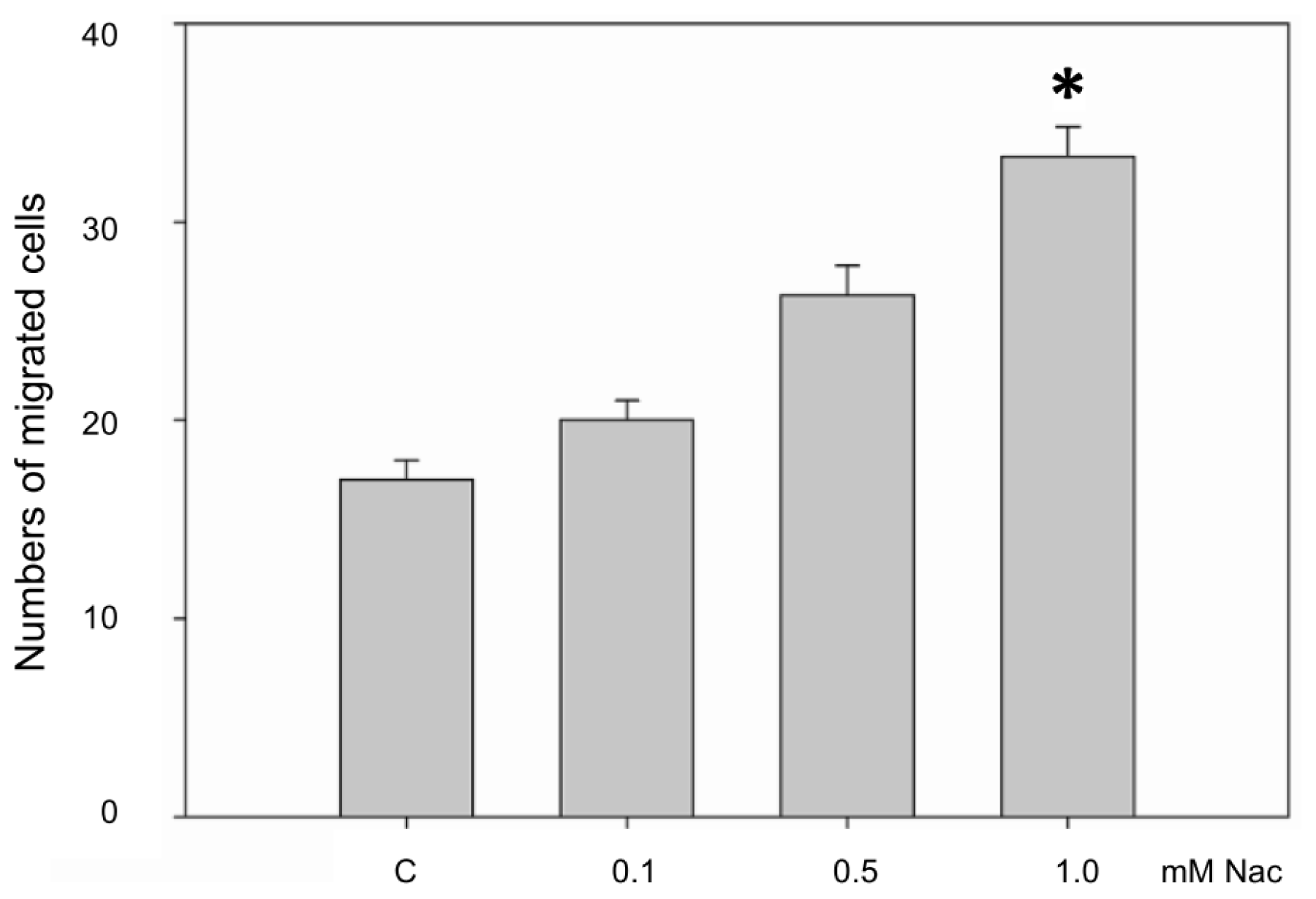
2.1.4. Nac Promoted the Migration of CCD-966SK Cells
2.1.5. Nac Promoted Matrixmetalloproteinase-1 (MMP-1) Expression in CCD-966SK Cells through Phosphatidylinositol 3-Kinase (PI3K) and Signal Transducer and Activator of Transcription 3 (Stat3) Signaling
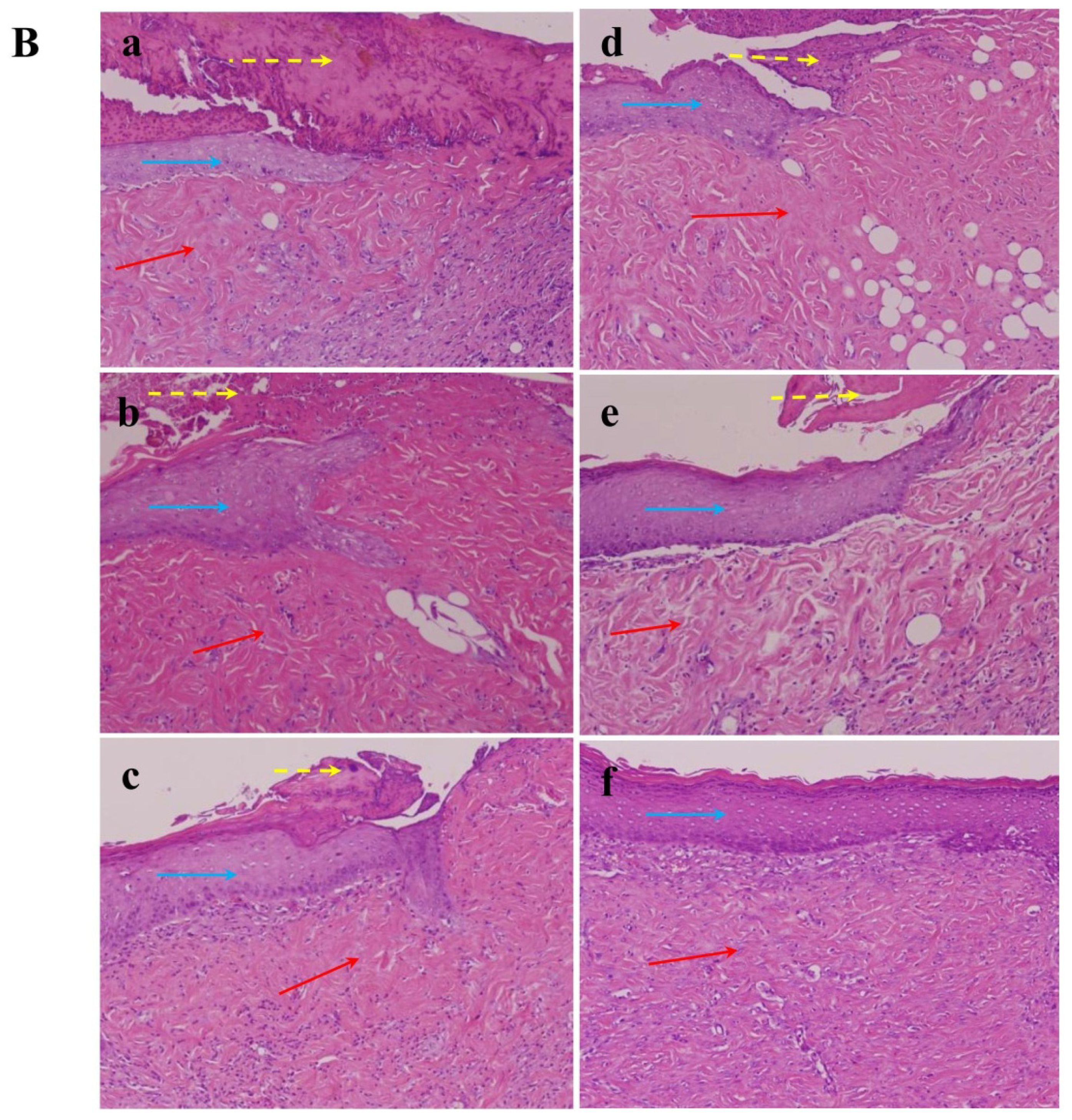
2.1.6. Effect of Nac on Healing of Wound Closure
2.2. Discussion
3. Experimental Section
3.1. Ethics Statement
3.2. Cell Culture and Preparation of Nac
3.3. Assessment of Cell Viability
3.4. Assessment of GSH
3.5. Scratch Wound Healing Assay
3.6. Boyden Chamber Migration Assay
3.7. Enzyme-Linked Immunosorbent (ELISA) Assay
3.8. Western Blotting Analysis
3.9. Wound Healing in Vivo Assay
3.9.1. Preparation of Nac
3.9.2. Burn Wound Model
3.9.3. Animal Treatment
3.10. Histopathological Study
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsConceived and designed the experiments: M.-L.T. Performed the experiments: M.-L.T., H.-P.H., J.-D.H. Analyzed the data: M.-L.T., H.-P.H., Y.-R.L. Contributed reagents/materials/analysis tools: M.-L.T., H.-P.H., J.-D.H., Y.-R.L., Y.-P.H., F.-J.L., H.-R.C. Wrote the paper: M.-L.T., H.-P.H., H.-R.C.
References
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev 2003, 83, 835–870. [Google Scholar]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med 1999, 341, 738–746. [Google Scholar]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev 2006, 19, 403–434. [Google Scholar]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; de Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol 2003, 66, 1499–1503. [Google Scholar]
- Pallardo, F.V.; Markovic, J.; Garcia, J.L.; Vina, J. Role of nuclear glutathione as a key regulator of cell proliferation. Mol. Asp. Med 2009, 30, 77–85. [Google Scholar]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A. N-Acetylcysteine—A safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol 2007, 7, 355–359. [Google Scholar]
- Kopke, R.D.; Jackson, R.L.; Coleman, J.K.; Liu, J.; Bielefeld, E.C.; Balough, B.J. NAC for noise: From the bench top to the clinic. Hear. Res 2007, 226, 114–125. [Google Scholar]
- Ozaydin, M.; Peker, O.; Erdogan, D.; Kapan, S.; Turker, Y.; Varol, E.; Ozguner, F.; Dogan, A.; Ibrisim, E. N-Acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study. Eur. Heart J 2008, 29, 625–631. [Google Scholar]
- Holt, S.; Marley, R.; Fernando, B.; Harry, D.; Anand, R.; Goodier, D.; Moore, K. Acute cholestasis-induced renal failure: Effects of antioxidants and ligands for the thromboxane A2 receptor. Kidney Int 1999, 55, 271–277. [Google Scholar]
- Toon, M.H.; Maybauer, M.O.; Greenwood, J.E.; Maybauer, D.M.; Fraser, J.F. Management of acute smoke inhalation injury. Crit. Care Resusc 2010, 12, 53–61. [Google Scholar]
- Sahib, A.S.; Al-Jawad, F.H.; Alkaisy, A.A. Effect of antioxidants on the incidence of wound infection in burn patients. Ann. Burn. Fire Disasters 2010, 23, 199–205. [Google Scholar]
- Deniz, M.; Borman, H.; Seyhan, T.; Haberal, M. An effective antioxidant drug on prevention of the necrosis of zone of stasis: N-Acetylcysteine. Burns 2013, 39, 320–325. [Google Scholar]
- Demir, E.O.; Cakmak, G.K.; Bakkal, H.; Turkcu, U.O.; Kandemir, N.; Demir, A.S.; Tascilar, O. N-Acetyl-cysteine improves anastomotic wound healing after radiotherapy in rats. J. Investig. Surg 2011, 24, 151–158. [Google Scholar]
- Sekundo, W.; Augustin, A.J.; Strempel, I. Topical allopurinol or corticosteroids and acetylcysteine in the early treatment of experimental corneal alkali burns: A pilot study. Eur J. Ophthalmol 2002, 12, 366–372. [Google Scholar]
- Demidova-Rice, T.N.; Geevarghese, A.; Herman, I.M. Bioactive peptides derived from vascular endothelial cell extracellular matrices promote microvascular morphogenesis and wound healing in vitro. Wound Repair Regen. 2011, 19, 59–70. [Google Scholar]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res 2010, 51, 224–229. [Google Scholar]
- Vaalamo, M.; Mattila, L.; Johansson, N.; Kariniemi, A.L.; Karjalainen-Lindsberg, M.L.; Kahari, V.M.; Saarialho-Kere, U. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J. Investig. Dermatol 1997, 109, 96–101. [Google Scholar]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar]
- Sano, S.; Chan, K.S.; DiGiovanni, J. Impact of Stat3 activation upon skin biology: A dichotomy of its role between homeostasis and diseases. J. Dermatol. Sci 2008, 50, 1–14. [Google Scholar]
- Litherland, G.J.; Elias, M.S.; Hui, W.; Macdonald, C.D.; Catterall, J.B.; Barter, M.J.; Farren, M.J.; Jefferson, M.; Rowan, A.D. Protein kinase C isoforms zeta and iota mediate collagenase expression and cartilage destruction via STAT3- and ERK-dependent c-fos induction. J. Biol. Chem 2010, 285, 22414–22425. [Google Scholar]
- De Vries, N.; de Flora, S. N-Acetyl-l-cysteine. J. Cell. Biochem 1993, S17F, 270–277. [Google Scholar]
- Kirichenko, A.K.; Bolshakov, I.N.; Ali-Rizal, A.E.; Vlasov, A.A. Morphological study of burn wound healing with the use of collagen-chitosan wound dressing. Bull. Exp. Biol. Med 2013, 154, 692–696. [Google Scholar]
- Gupta, A.; Singh, R.L.; Raghubir, R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol. Cell. Biochem 2002, 241, 1–7. [Google Scholar]
- Aldavood, S.J.; Behyar, R.; Sarchahi, A.A.; Rad, M.A.; Noroozian, I.; Ghamsari, S.M.; Sadeghi-Hashjin, G. Effect of acetylcysteine on experimental corneal wounds in dogs. Ophthalmic Res 2003, 35, 319–323. [Google Scholar]
- Khaksar, E.; Aldavood, S.J.; Abedi, G.R.; Sedaghat, R.; Nekoui, O.; Zamani-ahmadmahmudi, M. The effect of sub-conjunctival platelet-rich plasma in combination with topical acetylcysteine on corneal alkali burn ulcer in rabbits. Comp. Clin. Pathol 2013, 22, 107–112. [Google Scholar]
- Mackay, H.J.; Twelves, C.J. Targeting the protein kinase C family: Are we there yet? Nat. Rev. Cancer 2007, 7, 554–562. [Google Scholar]
- Yahata, Y.; Shirakata, Y.; Tokumaru, S.; Yamasaki, K.; Sayama, K.; Hanakawa, Y.; Detmar, M.; Hashimoto, K. Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation. J. Biol. Chem 2003, 278, 40026–40031. [Google Scholar]
- Takeda, K.; Akira, S. Multi-functional roles of Stat3 revealed by conditional gene targeting. Arch. Immunol. Ther. Exp 2001, 49, 279–283. [Google Scholar]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol 2008, 40, 1334–1347. [Google Scholar]
- Nascimento, E.G.; Sampaio, T.B.; Medeiros, A.C.; Azevedo, E.P. Evaluation of chitosan gel with 1% silver sulfadiazine as an alternative for burn wound treatment in rats. Acta Cirt. Bras 2009, 24, 460–465. [Google Scholar]





© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsai, M.-L.; Huang, H.-P.; Hsu, J.-D.; Lai, Y.-R.; Hsiao, Y.-P.; Lu, F.-J.; Chang, H.-R. Topical N-Acetylcysteine Accelerates Wound Healing in Vitro and in Vivo via the PKC/Stat3 Pathway. Int. J. Mol. Sci. 2014, 15, 7563-7578. https://doi.org/10.3390/ijms15057563
Tsai M-L, Huang H-P, Hsu J-D, Lai Y-R, Hsiao Y-P, Lu F-J, Chang H-R. Topical N-Acetylcysteine Accelerates Wound Healing in Vitro and in Vivo via the PKC/Stat3 Pathway. International Journal of Molecular Sciences. 2014; 15(5):7563-7578. https://doi.org/10.3390/ijms15057563
Chicago/Turabian StyleTsai, Min-Ling, Hui-Pei Huang, Jeng-Dong Hsu, Yung-Rung Lai, Yu-Ping Hsiao, Fung-Jou Lu, and Horng-Rong Chang. 2014. "Topical N-Acetylcysteine Accelerates Wound Healing in Vitro and in Vivo via the PKC/Stat3 Pathway" International Journal of Molecular Sciences 15, no. 5: 7563-7578. https://doi.org/10.3390/ijms15057563



