Docosahexaenoic Acid Supplementation during Pregnancy: A Potential Tool to Prevent Membrane Rupture and Preterm Labor
Abstract
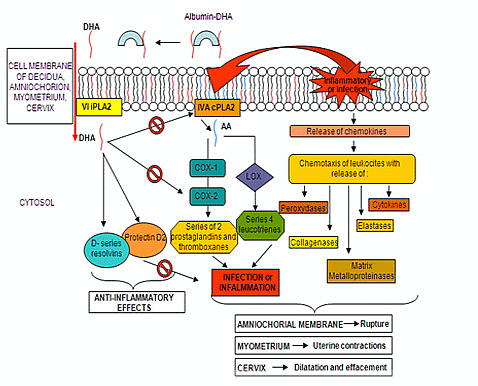
:1. Introduction
2. Pilot Experimental Section
3. Future Perspective
Acknowledgments
Conflicts of Interest
- Author ContributionsE.P., G.R., G.S. and F.S. performed the pilot clinical study and wrote the manuscript; M.M. contributed to the nutritional algorithms and verified the statistical content; F.D.C., P.V. contributed to the manuscript writing and to the verification of the entire study design; L.P. with F.S. coordinated the experimental and clinical framework and revised the entire manuscript.
References
- Serhan, C.N.; Chiang, N.; van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and proresolution lipid mediators. Nat. Rev. Immunol 2008, 22, 349–361. [Google Scholar]
- De Caterina, R.; Basta, G. n-3 Fatty acids and the inflammatory response—Biological background. Eur. Heart J. Suppl 2001, 3, D42–D49. [Google Scholar]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol 2011, 44, 203–215. [Google Scholar]
- Simopoulos, A.P.; Leaf, A.; Salem, N., Jr. Workshop statement on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 2000, 63, 119–121. [Google Scholar]
- Sanjurjo, P.; Matorras, R.; Perteagudo, L. Influence of fatty fish intake during pregnancy in the polyunsaturated fatty acids of erythrocyte phospholipids in the mother at labor and newborn infant. Acta Obstet. Gynecol. Scand 1995, 74, 594–598. [Google Scholar]
- Otto, S.J.; van Houwelingen, A.C.; Hornstra, G. The effect of supplementation with docosahexaenoic and arachidonic acid derived from single cell oils on plasma and erythrocyte fatty acids of pregnant women in the second trimester. Prostaglandins Leukot. Essent. Fatty Acids 2000, 63, 323–328. [Google Scholar]
- Gil-Sánchez, A.; Larqué, E.; Demmelmair, H.; Acien, M.I.; Faber, F.L.; Parrilla, J.J.; Koletzko, B. Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am. J. Clin. Nutr 2010, 92, 115–122. [Google Scholar]
- Lauritzen, L.; Hansen, H.S.; Jorgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res 2001, 40, 1–94. [Google Scholar]
- Montgomery, C.; Speake, B.K.; Cameron, A.; Sattar, N.; Weaver, L.T. Maternal docosahexaenoic acid supplementation and fetal accretion. Br. J. Nutr 2003, 90, 135–145. [Google Scholar]
- McCoy, M.G.; Sun, G.S.; Marchadier, D.; Maugeais, C.; Glick, J.M.; Rader, D.J. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res 2002, 43, 921–929. [Google Scholar]
- Hanebutt, F.L.; Demmelmair, H.; Schiessl, B.; Larque, E.; Koletzko, B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin. Nutr 2008, 27, 685–693. [Google Scholar]
- Parra, M.S.; Schnaas, L.; Meydani, M.; Perroni, E.; Martínez, S.; Romieu, I. Erythrocyte cell membrane phospholipid levels compared against reported dietary intakes of polyunsaturated fatty acids in pregnant Mexican women. Public Health Nutr 2002, 5, 931–937. [Google Scholar]
- Klingler, M.; Demmelmair, H.; Larque, E.; Koletzko, B. Analysis of FA contents in individual lipid fractions from human placental tissue. Lipids 2003, 38, 561–566. [Google Scholar]
- Chen, S.; Subbaiah, P.V. Phospholipid and fatty acid specificity of endothelial lipase: Potential role of the enzyme in the delivery of docosahexaenoic acid (DHA) to tissues. Biochim. Biophys. Acta 2007, 177, 1319–1328. [Google Scholar]
- Allen, K.G.; Harris, M.A. The role of n-3 fatty acids in gestation and parturition. Exp. Biol. Med 2001, 226, 498–506. [Google Scholar]
- Facchinetti, F.; Fazzio, M.; Venturini, P. Polyunsaturated fatty acids and risk of preterm delivery. Eur. Rev. Med. Pharmacol. Sci 2005, 9, 41–48. [Google Scholar]
- Olsen, S.F.; Osterdal, M.L.; Salvig, J.D.; Kesmodel, M.U.; Henriksen, T.B.; Hedegaard, M.; Secher, N.J. Duration of pregnancy in relation to seafood intake during early and mid pregnancy: Prospective cohort. Eur. J. Epidemiol 2006, 21, 749–758. [Google Scholar]
- Olsen, S.F.; Secher, N.J. Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: Prospective cohort study. Br. Med. J 2002, 324, 447–450. [Google Scholar]
- Olsen, S.F.; Grandjean, P.; Weihe, P.; Videro, T. Frequency of seafood intake in pregnancy as a determinant of birth weight: Evidence for a dose dependent relationship. J. Epidemiol. Community Health 1993, 47, 436–440. [Google Scholar]
- Olsen, S.F.; Sorensen, J.D.; Secher, N.J.; Hedegaard, M.; Henriksen, T.B.; Hansen, H.S.; Grant, A. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet 1992, 339, 1003–1007. [Google Scholar]
- Szajewska, H.; Horvath, A.; Koletzko, B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr 2006, 83, 1337–1344. [Google Scholar]
- Olsen, S.F.; Secher, N.J.; Tabor, A.; Weber, T.; Walker, J.J.; Gluud, C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. BJOG 2000, 107, 382–395. [Google Scholar]
- Horvath, A.; Koletzko, B.; Szajewska, H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Br. J. Nutr 2007, 98, 253–259. [Google Scholar]
- Green, J.T.; Orr, S.K.; Bazinet, R.P. The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. J. Lipid Res 2008, 49, 939–944. [Google Scholar]
- Rao, J.S.; Rapoport, S.I. Mood-stabilizers target the brain arachidonic acid cascade. Curr. Mol. Pharmacol 2009, 2, 207–214. [Google Scholar]
- Rao, J.S.; Ertley, R.N.; DeMar, J.C.; Rapoport, S.I.; Bazinet, R.P.; Lee, H.J. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol. Psychiatry 2007, 12, 151–157. [Google Scholar]
- Vincentini, O.; Quaranta, M.G.; Viora, M.; Agostoni, C.; Silano, M. Docosahexaenoic acid modulates in vitro the inflammation of celiac disease in intestinal epithelial cells via the inhibition of cPLA2. Clin. Nutr 2011, 30, 541–546. [Google Scholar]
- Gomez-Lopez, N.; Laresgoiti-Servitje, E.; Olson, D.M.; Estrada-Gutièrrez, G.; Vadillo-Ortega, F. The role of chemokines in term and premature rupture of the fetal membranes: A review. Biol. Reprod 2010, 82, 809–814. [Google Scholar]
- Van den Berg, J.J.M.; de Fouw, N.J.; Kuypers, F.A.; Roelofsen, B.; Houtsmuller, U.M.T.; Op den Kamp, J.A.F. Increased n-3 polyunsaturated fatty acid content of red blood cells from fish oil-fed rabbits increases in vitro lipid peroxidation, but decreases hemolysis. Free Radic. Biol. Med 1991, 11, 393–399. [Google Scholar]
- Mabile, L.; Piolot, A.; Boulet, L.; Fortin, L.J.; Doyle, N.; Rodriguez, C.; Davignon, J.; Blache, D.; Lussier-Cacan, S. Moderate intake of n-3 fatty acids is associated with stable erythrocyte resistance to oxidative stress in hypertriglyceridemic subjects. Am. J. Clin. Nutr 2001, 74, 449–456. [Google Scholar]
- Hashimoto, M.; Hossain, S.; Shimada, T.; Shido, O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid β-infused rats is associated with increased synaptosomal membrane fluidity. Clin. Exp. Pharmacol. Physiol 2006, 33, 934–939. [Google Scholar]
- Palozza, P.; Sgarlata, E.; Luberto, C.; Piccioni, E.; Anti, M.; Marra, G.; Armelao, F.; Franceschelli, P.; Bartoli, G.M. n-3 Fatty acids induce oxidative modifications in human erythrocytes depending on dose and duration of dietary supplementation. Am. J. Clin. Nutr 1996, 64, 297–304. [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Assessment of risk factors for preterm birth. Clinical management guidelines for obstetrician-gynecologists. Number 31, October 2001 (Replaces Technical Bulletin number 206, June 1995; Committee Opinion number 172, May 1996 ; Committee Opinion number 187, September 1997; Committee Opinion number 198, February 1998; and Committee Opinion number 251, January 2001. Obstet. Gynecol 2001, 98, 709–716.
- EMedicine from WebMD, Medscape’s Continually Updated Clinical Reference. Available online: http://emedicine.medscape.com/article/261137-overview accessed on 20 February 2013.
- Seaward, P.G.; Hannah, M.E.; Myhr, T.L.; Farine, D.; Ohlsson, A.; Wang, E.E.; Haque, K.; Weston, J.A.; Hewson, S.A.; Ohel, G.; et al. International multicentre term prelabor rupture of membranes study: Evaluation of predictors of clinical chorioamnionitis and postpartum fever in patients with prelabor rupture of membranes at term. Am. J. Obstet. Gynecol 1997, 177, 1024–1029. [Google Scholar]
- Hannah, M.E.; Ohlsson, A.; Farine, D.; Hewson, S.A.; Hodnett, E.D.; Myhr, T.L.; Wang, E.E.; Weston, J.A.; Willan, A.R. Induction of labor compared with expectant management for prelabor rupture of the membranes at term. N. Engl. J. Med 1996, 334, 1005–1010. [Google Scholar]
- Mercer, B.M.; Arheart, K.L. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet 1995, 346, 1271–1279. [Google Scholar]
- Schucker, J.L.; Mercer, B.M. Midtrimester premature rupture of the membranes. Semin. Perinatol 1996, 20, 389–400. [Google Scholar]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction 2014. [Google Scholar] [CrossRef]
- Quinlivan, J.A.; Pakmehr, S. Fish oils as a population based strategy to reduce early preterm birth. Reprod. Syst. Sex. Disord 2013. [Google Scholar] [CrossRef]
- Mozurkewich, E.L.; Clinton, C.M.; Chilimigras, J.L.; Hamilton, S.E.; Allbaugh, L.J.; Berman, D.R.; Marcus, S.M.; Romero, V.C.; Treadwell, M.C.; Keeton, K.L.; et al. The Mothers, Omega-3, and Mental Health Study: A double-blind, randomized controlled trial. Am. J. Obstet. Gynecol 2013, 208, 313.e1–313.e9. [Google Scholar]
- Deligiannidis, K.M.; Freeman, M.P. Complementary and alternative medicine therapies for perinatal depression. Best Pract. Res. Clin. Obstet. Gynaecol 2014, 28, 85–95. [Google Scholar]
- Strøm, M.; Mortensen, E.L.; Halldorsson, T.I.; Thorsdottir, I.; Olsen, S.F. Fish and long-chain n-3 polyunsaturated fatty acid intakes during pregnancy and risk of postpartum depression: A prospective study based on a large national birth cohort. Am. J. Clin. Nutr 2009, 90, 149–155. [Google Scholar]
- Skotheim, S.; Braarud, H.C.; Høie, K.; Markhus, M.W.; Malde, M.K.; Graff, I.E.; Berle, J.Ø.; Stormark, K.M. Subclinical levels of maternal depression and infant sensitivity to social contingency. Infant Behav. Dev 2013, 36, 419–426. [Google Scholar]
- Crawford, M.A.; Broadhurst, C.L. The role of docosahexaenoic and the marine food web as determinants of evolution and hominid brain development: The challenge for human sustainability. Nutr. Health 2012, 21, 17–39. [Google Scholar]
- Loomans, E.M.; van den Bergh, B.R.; Schelling, M.; Vrijkotte, T.G.; van Eijsden, M. Maternal long-chain polyunsaturated fatty acid status during early pregnancy and children’s risk of problem behavior at age 5–6 years. J. Pediatr 2014, 164, 762–768. [Google Scholar]
- Rogers, L.K.; Valentine, C.J.; Keim, S.A. DHA supplementation: Current implications in pregnancy and childhood. Pharmacol. Res 2013, 70, 13–19. [Google Scholar]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P. DOMInO Investigative Team. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar]
- Lumia, M.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Erkkola, M.; Uusitalo, L.; Niinistö, S.; Kenward, M.G.; Ilonen, J.; Simell, O.; et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr. Allergy Immunol 2011, 22, 827–835. [Google Scholar]
- Pistiner, M.; Gold, D.R.; Abdulkerim, H.; Hoffman, E.; Celedon, J.C. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J. Allergy Clin. Immunol 2008, 122, 274–279. [Google Scholar]
- Makrides, M.; Gunaratne, A.W.; Collins, C.T. Dietary n-3 LC-PUFA during the perinatal period as a strategy to minimize childhood allergic disease. Nestle Nutr. Inst. Workshop Ser 2013, 77, 155–162. [Google Scholar]


| Characteristic | DHA group n = 129 | Placebo group n = 126 |
|---|---|---|
| Age (years) Mean ± SD | 30.86 ± 4.18 | 29.92 ± 4.80 |
| BMI (Kg/m2) Mean ± SD | 23.24 ± 1.60 | 22.65 ± 2.11 |
| Parity (%) | ||
| 0 | 46 (36) | 50 (40) |
| 1 | 83 (64) | 76 (60) |
| Amniocentesis (%) | ||
| Yes | 20 (16) | 13 (10) |
| No | 109 (84) | 113 (90) |
| Genital infection with effective treatment (%) | ||
| Yes | 9 (7) | 13 (10) |
| No | 120 (93) | 113 (90) |
| FAs | Erythrocyte FAs (nmol/mL) | Plasma FAs (nmol/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DHA group | Placebo group | p | DHA group | Placebo group | p | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| 17th week | n = 129 | n = 126 | n = 129 | n = 126 | ||||||
| 20:3 ω-6 * | 126 | 9.06 | 127 | 0.52 | 0.31 | 247 | 15.49 | 248 | 15.71 | 0.40 |
| 20:4 ω-6 | 337 | 28.47 | 336 | 28.58 | 0.84 | 237 | 13.35 | 235 | 13.74 | 0.37 |
| 20:5 ω-3 | 8 | 4.13 | 8 | 4.73 | 0.14 | 25 | 4.95 | 26 | 5.48 | 0.13 |
| 22:6 ω-3 | 149 | 21.16 | 148 | 20.47 | 0.70 | 135 | 7.89 | 134 | 7.81 | 0.41 |
| Total ω-3 FAs | 156 | 21.03 | 156 | 21.44 | 0.95 | 159 | 8.54 | 160 | 9.62 | 0.86 |
| Total ω-6 FAs | 463 | 30.40 | 463 | 29.41 | 0.91 | 483 | 20.46 | 483 | 20.82 | 0.96 |
| ω-6/ω-3 | 3.00 | 0.37 | 3.02 | 0.47 | 0.70 | 3.04 | 0.21 | 3.04 | 0.21 | 0.96 |
| 25th week | n = 129 | n = 126 | n = 129 | n = 126 | ||||||
| 20:3 ω-6 | 147 | 41.08 | 139 | 31.93 | 0.08 | 338 | 16.13 | 341 | 12.80 | 0.16 |
| 20:4 ω -6 | 248 | 12.16 | 275 | 10.62 | 0.00 | 269 | 16.72 | 277 | 15.95 | 0.00 |
| 20:5 ω-3 | 11 | 6.19 | 11 | 4.41 | 0.19 | 28 | 7.06 | 27 | 7.80 | 0.27 |
| 22:6 ω-3 | 201 | 13.10 | 186 | 18.12 | 0.00 | 220 | 14.97 | 189 | 29.29 | 0.00 |
| Total ω-3 FAs | 213 | 14.43 | 196 | 18.80 | 0.00 | 248 | 17.65 | 217 | 31.05 | 0.00 |
| Total ω-6 FAs | 395 | 46.76 | 510 | 33.63 | 0.00 | 607 | 27.38 | 618 | 21.89 | 0.00 |
| ω-6/ω-3 | 1.86 | 0.24 | 2.13 | 0.27 | 0.00 | 2.45 | 0.19 | 2.91 | 0.43 | 0.00 |
| 38th week | n = 124 | n = 115 | n = 124 | n = 115 | ||||||
| 20:3 ω-6 | 110 | 11.10 | 110 | 11.19 | 0.96 | 304 | 6.36 | 303 | 6.85 | 0.27 |
| 20:4 ω -6 | 224 | 26.75 | 252 | 19.62 | 0.00 | 255 | 18.14 | 261 | 15.24 | 0.01 |
| 20:5 ω-3 | 6 | 4.18 | 5 | 3.41 | 0.12 | 31 | 6.10 | 30 | 5.91 | 0.26 |
| 22:6 ω-3 | 174 | 12.71 | 126 | 17.76 | 0.00 | 178 | 9.04 | 137 | 20.58 | 0.00 |
| Total ω-3 FAs | 179 | 13.98 | 130 | 17.96 | 0.00 | 209 | 11.54 | 167 | 20.82 | 0.00 |
| Total ω-6 FAs | 334 | 30.77 | 362 | 21.30 | 0.00 | 559 | 20.06 | 564 | 18.64 | 0.04 |
| ω-6/ω-3 | 1.87 | 0.22 | 2.83 | 0.40 | 0.00 | 2.68 | 0.17 | 3.43 | 0.45 | 0.00 |
| ROM | DHA group n = 129 | Placebo group n = 126 | Total n = 255 | p value |
|---|---|---|---|---|
| pPROM | 1 | 4 | 5 | 0.02 |
| PROM | 5 | 12 | 17 | 0.02 |
| Total (%) | 6 (4.7) | 16 (1.3) | 22 (9) | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pietrantoni, E.; Del Chierico, F.; Rigon, G.; Vernocchi, P.; Salvatori, G.; Manco, M.; Signore, F.; Putignani, L. Docosahexaenoic Acid Supplementation during Pregnancy: A Potential Tool to Prevent Membrane Rupture and Preterm Labor. Int. J. Mol. Sci. 2014, 15, 8024-8036. https://doi.org/10.3390/ijms15058024
Pietrantoni E, Del Chierico F, Rigon G, Vernocchi P, Salvatori G, Manco M, Signore F, Putignani L. Docosahexaenoic Acid Supplementation during Pregnancy: A Potential Tool to Prevent Membrane Rupture and Preterm Labor. International Journal of Molecular Sciences. 2014; 15(5):8024-8036. https://doi.org/10.3390/ijms15058024
Chicago/Turabian StylePietrantoni, Emanuela, Federica Del Chierico, Giuliano Rigon, Pamela Vernocchi, Guglielmo Salvatori, Melania Manco, Fabrizio Signore, and Lorenza Putignani. 2014. "Docosahexaenoic Acid Supplementation during Pregnancy: A Potential Tool to Prevent Membrane Rupture and Preterm Labor" International Journal of Molecular Sciences 15, no. 5: 8024-8036. https://doi.org/10.3390/ijms15058024