KRAS/BRAF Analysis in Ovarian Low-Grade Serous Carcinoma Having Synchronous All Pathological Precursor Regions
Abstract
:1. Introduction
2. Results
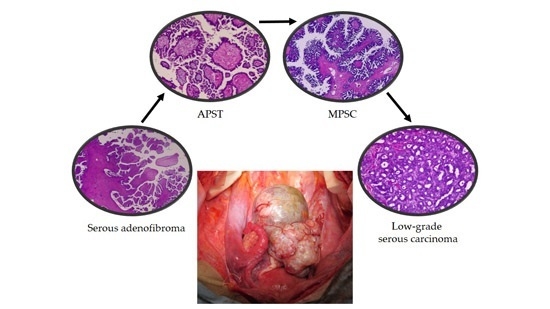
2.1. Pathological Findings
2.1.1. Atypical Proliferative Serous Tumor (APST)
2.1.2. Noninvasive Noninvasive Micropapillary Serous Borderline Tumor (MPSC)
2.1.3. Low-Grade Serous Carcinoma (Invasive Focus of MPSC)
2.2. Genetic Analysis of Distinct Tumor Regions
3. Discussion
4. Materials and Methods
4.1. Tissue Sample
4.2. Laser Capture Microdissection and DNA Extraction
4.3. Sequence Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global canceer statistics. Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Singer, G.; Kurman, R.J.; Chang, H.W.; Cho, S.K.R.; Shih, I.M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am. J. Pathol. 2002, 160, 1223–1228. [Google Scholar] [CrossRef]
- Shih, I.M.; Kurman, R.J. Molecular pathogenesis of ovarian borderline tumors: New insights and old challenges. Clin. Cancer Res. 2005, 11, 7273–7279. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.J.; Kurman, R.J.; Wang, M.; Oldt, R.; Wang, B.G.; Berman, D.M.; Shih, I.M. Molecular genetic analysis of ovarian serous cystadenomas. Lab. Investig. 2004, 84, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Kurman, R.J.; Dehari, R.; Wang, T.L.; Shih, I.M. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res. 2004, 64, 6915–6918. [Google Scholar] [CrossRef] [PubMed]
- Malpica, A.; Deavers, M.T.; Lu, K.; Bodurka, D.C.; Atkinson, E.N.; Gershenson, D.M.; Silva, E.G. Grading ovarian serous carcinoma using a two-tier system. Am. J. Surg. Pathol. 2004, 28, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Smith Sehdev, A.E.; Sehdev, P.S.; Kurman, R.J. Noninvasive and invasive micropapillary (low-grade) serous carcinoma of the ovary: A clinicopathologic analysis of 135 cases. Am. J. Surg. Pathol. 2003, 27, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.A.; Longacre, T.A.; Prat, J.; Kohn, E.C.; Soslow, R.A.; Ellenson, L.H.; Malpica, A.; Stoler, M.H.; Kurman, R.J. Serous borderline (low malignant potential, atypical proliferative) ovarian tumor: Workshop perspectives. Hum. Pathol. 2004, 35, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Longacre, T.A.; McKenney, J.K.; Tazalaar, H.D.; Kempson, R.L.; Hendrickson, M.R. Ovarian serous tumors of low malignant potential (borderline tumors): Outcome-based study of 276 patients with long-term (> or = 5-year) follow-up. Am. J. Surg. Pathol. 2005, 29, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Singer, G.; Oldt, R., 3rd; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih, I.M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Singer, G.; Shih, I.M.; Truskinovsky, A.; Umudum, H.; Kurman, R.J. Mutational analysis of K-ras segregates ovarian serous carcinomas into two types: Invasive MPSC (low-grade tumor). Int. J. Gynecol. Pathol. 2003, 22, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.T.; Deavers, M.T.; Sun, C.C.; Kwan, S.Y.; Kuo, E.; Malpica, A.; Mok, S.C.; Gershenson, D.M.; Wong, K.K. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J. Pathol. 2013, 231, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Ryland, G.L.; Hunter, S.M.; Doyle, M.A.; Caramia, F.; Li, J.; Rowley, S.M.; Christie, M.; Allan, P.E.; Stephens, A.N.; Bowtell, D.D.; et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med. 2015, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Wang, T.L.; Kurman, R.J.; Nakayama, K.; Velculescu, V.E.; Vogelstein, B.; Kinzler, K.W.; Papadopoulos, N.; Shih, I.M. Low-grade serous carcinomas of the ovary contain very few point mutations. J. Pathol. 2012, 226, 413–420. [Google Scholar] [CrossRef] [PubMed]




| Gene | Serous Adenofibroma | APST | Noninvasive MPSC | Low-Grade Serous Carcinoma | Noninvasive Implant | Lymph Node Associated with APST |
|---|---|---|---|---|---|---|
| KRAS | WT | WT | WT | WT | NA | NA |
| BRAF | WT | WT | WT | WT | NA | NA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, K.; Nakayama, K.; Ishibashi, T.; Ishikawa, N.; Ishikawa, M.; Katagiri, H.; Minamoto, T.; Sato, E.; Sanuki, K.; Yamashita, H.; et al. KRAS/BRAF Analysis in Ovarian Low-Grade Serous Carcinoma Having Synchronous All Pathological Precursor Regions. Int. J. Mol. Sci. 2016, 17, 625. https://doi.org/10.3390/ijms17050625
Nakamura K, Nakayama K, Ishibashi T, Ishikawa N, Ishikawa M, Katagiri H, Minamoto T, Sato E, Sanuki K, Yamashita H, et al. KRAS/BRAF Analysis in Ovarian Low-Grade Serous Carcinoma Having Synchronous All Pathological Precursor Regions. International Journal of Molecular Sciences. 2016; 17(5):625. https://doi.org/10.3390/ijms17050625
Chicago/Turabian StyleNakamura, Kohei, Kentaro Nakayama, Tomoka Ishibashi, Noriyoshi Ishikawa, Masako Ishikawa, Hiroshi Katagiri, Toshiko Minamoto, Emi Sato, Kaori Sanuki, Hitomi Yamashita, and et al. 2016. "KRAS/BRAF Analysis in Ovarian Low-Grade Serous Carcinoma Having Synchronous All Pathological Precursor Regions" International Journal of Molecular Sciences 17, no. 5: 625. https://doi.org/10.3390/ijms17050625