1. Introduction
Growth hormone (GH) is essential for body growth during childhood and continues to stimulate anabolic processes in adults. GH exerts its anabolic effects largely indirectly via stimulation of insulin-like growth factor-1 (IGF1) production. Components of the GH–IGF1 axis make an important contribution to the development, function, and proliferation of different tissues [
1]. Moreover, acting in endocrine/paracrine/autocrine manner, GH binds to the specific GH receptor (GHR), which may induce two downstream pathways: (i) the JAK2-STAT5 pathway that in turn leads to up-regulation of GH responsive elements; and (ii) the RAS/MAPK/ERK pathway leading to enhanced transcriptional activity of GH-regulated genes [
1]. The classical examples of IGF1-independent actions mediated through GHR are the proliferation of chondrocyte stem cells at bone growth plate [
2], the fusion of myoblasts with nascent myotubes to increase muscle fiber size [
3] or direct stimulation of neural stem cells to proliferate [
4]. Additionally, GHR has been shown to be expressed in different mature hematopoietic cells [
5].
Recent studies have also focused on the anti-apoptotic effects of GH. Apoptosis is the most common, gene-directed form of programmed cell death that contributes to different physiologic and pathologic processes. Apoptosis also plays a key role in controlling the homeostasis of the hematopoietic system. Several lines of evidence indicate that different hormones play a role as regulators of eukaryotic cell death [
6]. Likewise, several studies revealed diverse biological actions of GH in different types of cells, including prevention of apoptosis in mature hematopoietic and immune-related cells. For instance, GH has been shown to prevent apoptosis of immune-related cells through different molecular mechanisms, such as down-regulation of pro-apoptotic Fas Ligand in human neutrophils [
7], rising the expression of the anti-apoptotic Bcl-2 in primary monocytes, promonocytic cell line [
8,
9] and human lymphocytes [
10] or inhibition of caspase-3 activity in human T and B lymphocyte cell lines [
8,
9]. On the other hand, GH has been shown to inhibit the production of pro-inflammatory cytokines in many cells, shifting the balance in favor of anti-inflammatory cytokines, which may potentially play an important role in prevention of apoptosis [
11]. Importantly, the anti-apoptotic GH effects have also been observed in conditions characterized by increased local GH production. Arnold and Weigent found that GH-overexpressing T cell lymphoma can reduce chemically-induced apoptosis via increased expression of BCL-2, with concurrent decrease in expression of pro-apoptotic molecules [
12]. In contrast, the same cells depleted of GH were characterized by the increased apoptosis rate. These results strongly indicate that GH plays an important role in the survival of hematopoietic cells exposed to stressful stimuli. However, most of the studies on the apoptotic activity in selected types of hematopoietic cells are based on in vitro conditions. Data about alterations of programmed cell death process following GH administration in hematopoietic system in vivo are very scarce. Decker et al. observed that the GH treatment of elderly subjects undergoing vascular repair considerably reduced apoptosis in circulating neutrophils in vivo [
13].
Patients with GH deficiency (GHD) can display abnormalities in hematopoietic cells circulating in their peripheral blood (PB), including decreased red blood cell mass and quantity [
14,
15]. Especially, GHD patients frequently suffer from certain types of anemia, including “normocytic-normochromic” anemia [
15,
16]. The number of white blood cells in GHD patients is frequently decreased too [
17]. These findings may be related in part to apoptosis activity in GHD. It was observed that physiological and biochemical abnormalities found in GHD may be modified by GH therapeutic supplementation (GH-TS) [
18]. However, data regarding the molecular effects of GH on modulation of cellular processes in hematopoietic progenitor cells are very limited and the biological activities of GH supplementation in these cells need further studies.
In the present study, we investigated the in vivo effects of GH-TS on apoptosis in CD34+ cells enriched in hematopoietic progenitor cells (HPCs) collected from children with isolated GHD. CD34+ cells were examined by different methods for apoptosis activity, including Annexin-V labeling, terminal deoxynucleotidyl transferase-mediated dUTP labeling (TUNEL), and analysis of the expression of apoptosis-related molecules, i.e., BCL-2, BCL-xL, BAX and Caspase-3. Finally, the global gene expression profiles in collected cells were analyzed using RNA microarrays.
3. Discussion
In the last decade, GH therapy has yielded favorable results for childhood growth disorders and adult GHD; however, little is known about the particular functions in the hematopoietic system of GHD patients, including apoptosis, in course of GH therapy. GH initiates a cascade of biological effects by binding to the GH receptor (GHR), what induces direct phosphorylation of target signaling proteins ultimately leading to changes in gene expression [
1]. Although a number of GH-regulated genes have been identified, genes relating GH to programmed cell death in hematopoietic stem and progenitor cells are not comprehensively known. Given that patients with GHD do not produce significant amounts of GH and GH therapy is the main source of hormone in their organism, we have chosen this disease as an in vivo model for identifying apoptosis-related genes that can be modulated in human HPCs by exogenous GH. Accordingly, we sought to investigate the effects of long-term GH treatment on apoptosis in hematopoietic system of children with severe GHD. The current study, to the best of our knowledge, is the first such study to evaluate the early and late apoptosis and its main control mechanisms in CD34+ cells collected from GHD children.
Apoptosis is a basic process, which contributes to the regulation of cell lifespan. In this notion, GH/GHR axis has been shown to be important regulator of apoptosis [
19]. Apoptosis plays also a key role in the controlling of the hematopoietic cell homeostasis. Several hormones, such as prolactin or glucocorticoids have been shown to play a role in cell survival and apoptosis of the hematopoietic cells in vitro [
20]. Moreover, we previously have found that thyroid hormones are able to affect apoptosis in normal human HPCs in vitro, and in patients suffering from hyperthyroidism [
21,
22]. There is a substantial empirical evidence to suggest that GH has also protective effect on human hematopoietic cells. This hypothesis is based on the observations of the GH-dependent increase in the number and production rate of different blood-borne cell populations in different cohorts of GHD patients treated with GH, including white or red blood cells (RBC). Christ et al. observed that a GH treatment over 3 months increased significantly the RBC counts in adult GHD patients [
14]. Similarly, Bergamaschi et al. evaluated several GHD patients diagnosed with normochromic, normocytic anemia, and observed the increase in RBC counts, which restored their normal levels after 12 months of GH treatment [
19]. The changes in RBC indices, hemoglobin concentration or hematopoietic precursor cell levels following GH therapy have also been observed in different groups of patients with and without GH deficiency [
23,
24,
25]. Interestingly, when GH deficiency is associated with multiple pituitary hormone deficiencies, the pathological deficit in erythropoiesis was not corrected until GH treatment was started [
15]. Likewise, the size of different leukocyte populations was significantly increased during GH administration, indicating an important role of GH in hematopoietic system functions [
17,
26,
27]. We postulate here that the significantly decreased numbers of RBCs in GHD patients reported by other groups might be associated with the considerably higher percentage of apoptotic cells, and the increased BAX/BCL-2 mRNA and protein ratios, which were detected by us in CD34+ cells from untreated GHD patients compared to their controls. In contrast, diminished RBC deficiency in course of GH-TS may reflect either a stimulatory effect of GH on cell survival or/and inhibition of apoptosis mechanism. Therefore, it is crucial to analyze the involvement of GH in the regulation of apoptosis in CD34+ hematopoietic cells from GHD patients.
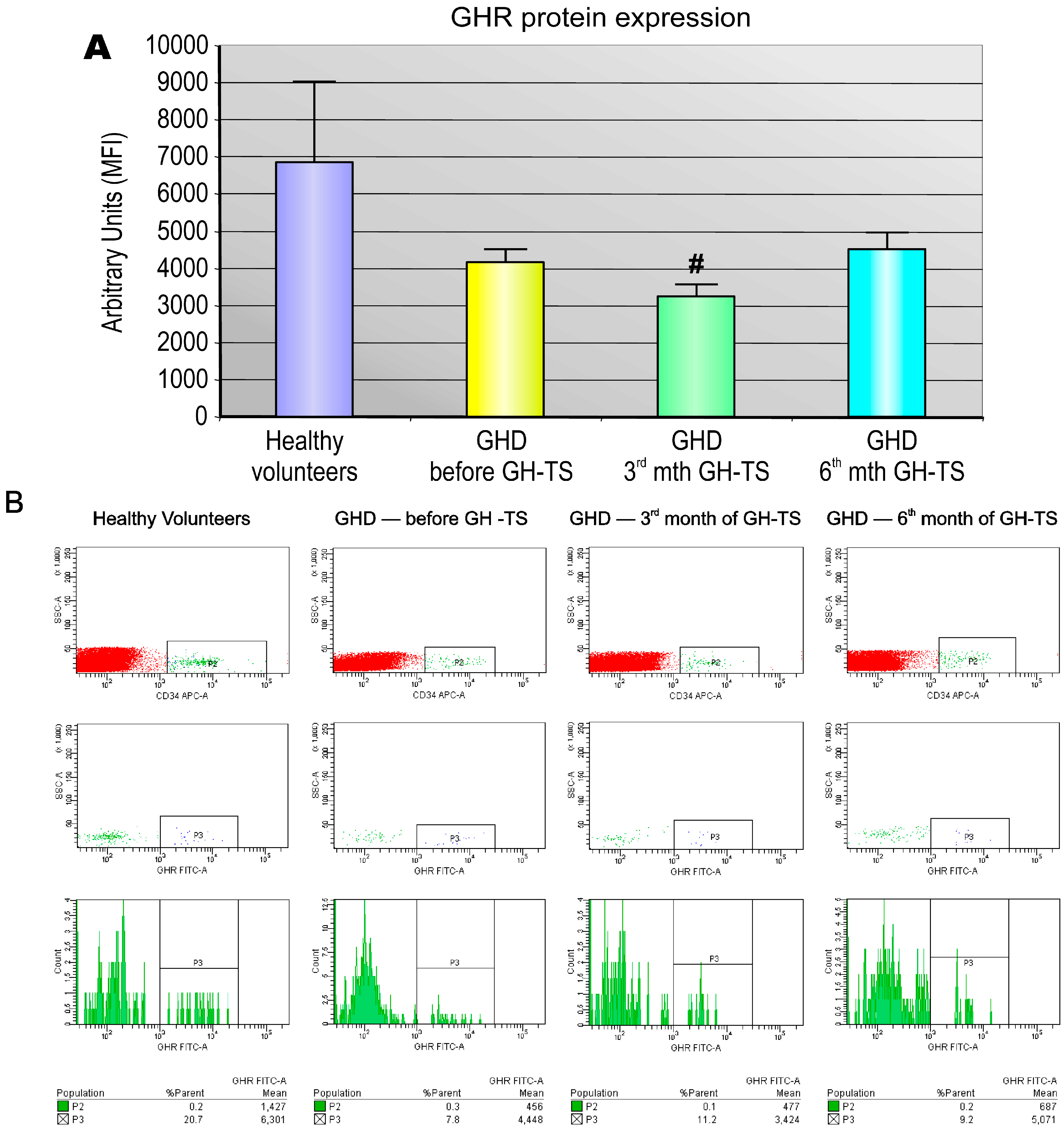
Several investigators reported that GHRs are expressed in diverse types of human mature hematopoietic cells, predominantly in monocytes/macrophages or B lymphocytes, but also in neutrophils, natural killer cells and T lymphocytes [
28,
29,
30], suggesting a direct action of GH on these cells. Cool et al. also revealed that number of CD34+ cells in bone marrow of GHR deficient mice is markedly decreased relative to controls [
31]. This may strongly indicate that GH has a potential influence on the development of hematopoietic progenitors in the bone marrow. However, there is lack of studies providing the evidence that active GHR is expressed in CD34+ cells circulating in GHD children. We demonstrated using IF technique that CD34+ cells from GHD patients express GHR protein on cell surface. Therefore, these cells are possible targets for GH-induced modulation of cellular functions. Similar results were reported by Gagnerault et al., who showed that all hematopoietic lineages of the murine bone marrow expressed GHR, however, at various levels [
5]. We found that CD34+ cells from GHD children treated with GH for 3 months expressed significantly lower quantities of GHR protein compared to controls. This finding may be explained by the possibility that the produced GHR molecules were internalized into the cytoplasm in GH-activated cells as the form of the response to exogenously administered GH. In addition, the functional activity of GHR expressed in CD34+ cells was assessed. Our studies have shown that exogenous GH effectively activates GHR and the subsequent phosphorylation of STAT-5 protein. We also found that GHD state resulted in significant reduction of phosphorylated form of STAT-5 in CD34+ cells, which could be reversed by GH-TS. This result suggests that biological axis formed by exogenous GH and GHRs in CD34+ cells from GHD patients is fully functional and may induce the intracellular activation of GH/GHR-related signaling pathways during the long-term GH-TS, which therefore may contribute to the modulation of hematopoiesis in GHD patients.
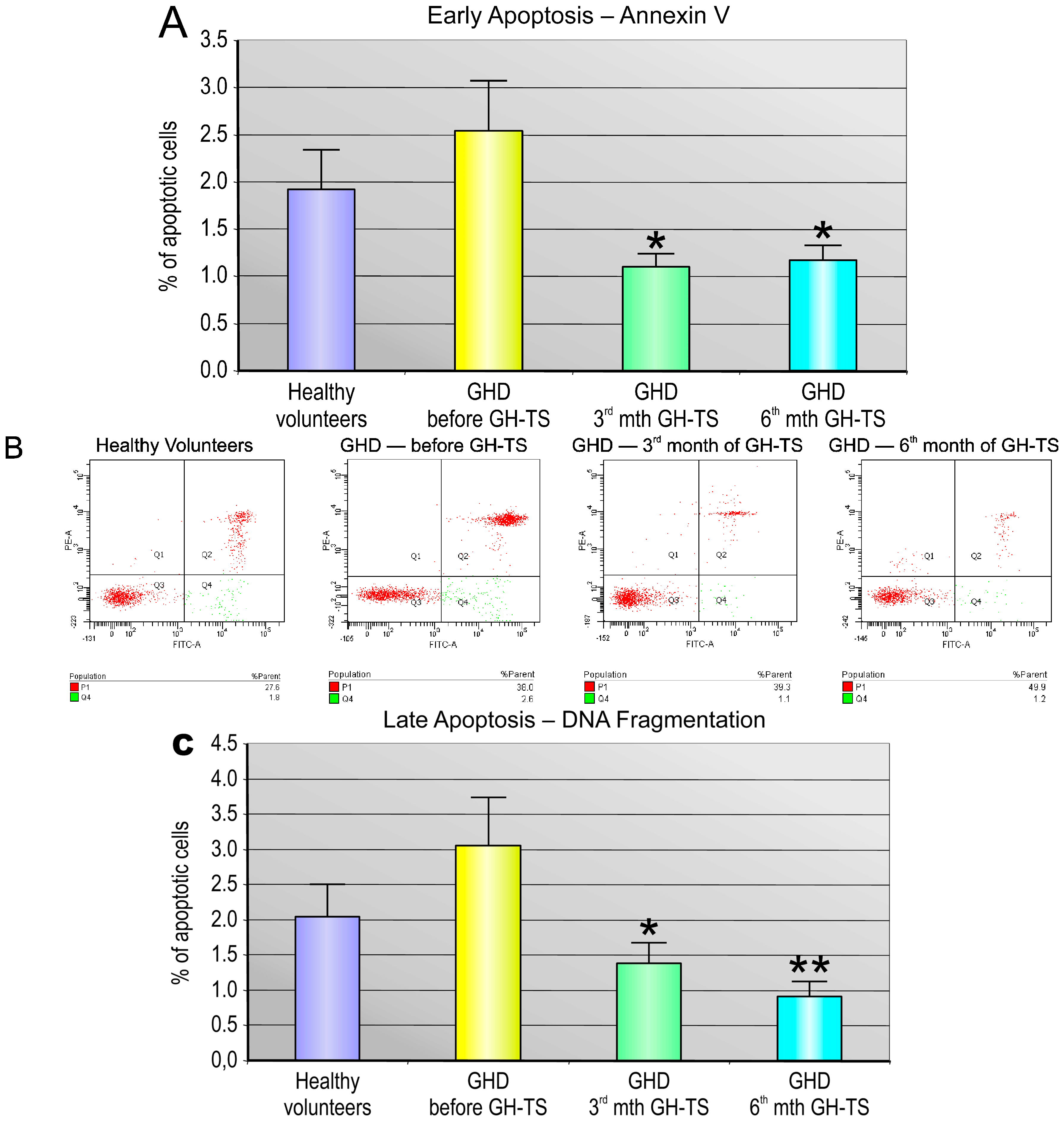
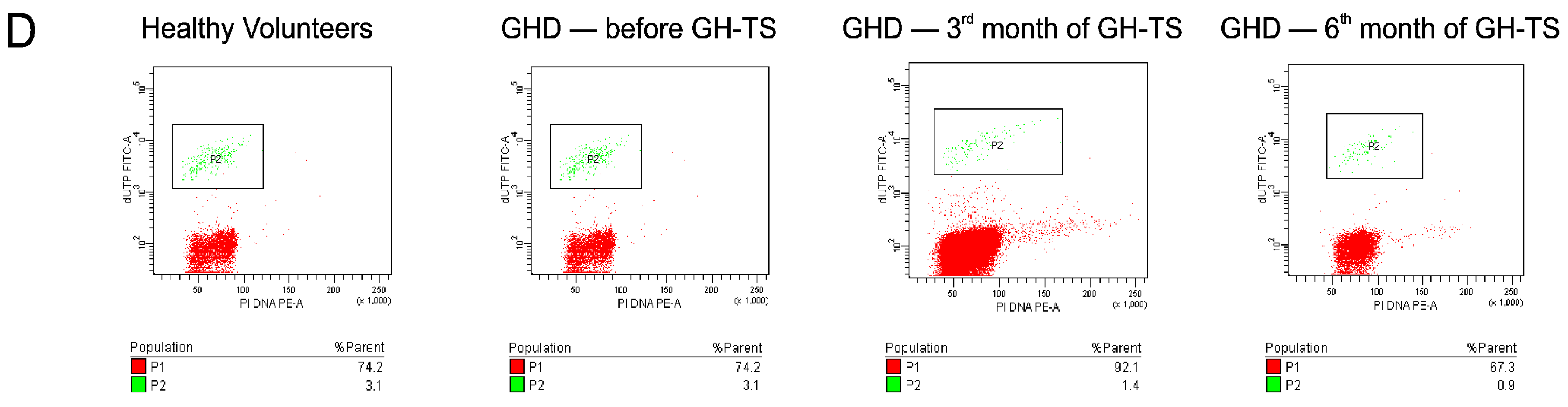
In general, we found that CD34+ cells from GHD patients had different apoptosis rate compared to that of controls. Particularly, we demonstrated that these cells collected from GHD patients examined prior to the GH treatment exhibited considerably increased levels of apoptosis at both early and late phases compared to their controls. Similar results were reported by Kennedy et al., who showed an increased apoptosis rate in livers of Ames dwarf mice stably deficient in GH, suggesting that GH plays an important role in the cell survival [
32]. Besides, Arnold and Weigent have shown that treatment of the lymphoma cells with antisense deoxyoligonucleotides to specifically block endogenous GH expression augmented cell apoptosis through the decreased expression of apoptosis inhibitors resulting in the DNA fragmentation [
12]. In contrast, the treatment with GH resulted in a significant decrease of the percentage of apoptotic CD34+ cells from GHD patients in our study. Similar results were reported by Baixeras et al. [
33] and by Haeffner et al. [
8], who have confirmed that GH treatment can inhibit apoptosis in hematopoietic cells. GH has also been shown to be anti-apoptotic agent in other types of cells, including myoblasts, intestinal and ovarian granulosa cells [
34,
35,
36]. The observed reduction of apoptosis due to the GH treatment may be explained by several mechanisms. The multiple actions of GH are initiated by binding to GHR. It activates the tyrosine kinase JAK-2, which is the major pathway whereby GH exerts its cellular effects [
37]. The JAK-2-mediated signal transduction pathway has been shown to mediate induction of BCL-2 and NF-kB resulting in delay of hematopoietic cell death [
6]. In addition to activating the RAS/MAPK pathway, GH can increase the homeo-box A1-dependent expression of BCL-2 as reported in human mammary carcinoma cells [
38]. It is, therefore, conceivable that the up-regulation of the BCL-2 protein may be an essential mechanism by which GH directly exerts its protective effect on different cells. Likewise, we observed a significant increase in the Bcl-2 gene expression induced by GH therapy in cells of GHD patients, which was followed by clearly augmented production of Bcl-2 protein as detected by western blot analysis. In contrast, GH treatment did not affect the reported BCL-2 expression in blastocysts, but reduced the expression of the pro-apoptotic BAX protein [
39]. Besides, GH treatment inhibited apoptosis in human leukemic cells and Chinese hamster ovary cells through stimulation of the anti-apoptotic serine kinase AKT, leaving unchanged the BCL-2 levels [
40]. The GH-dependent activation of AKT has been shown to be dependent on the presence of the JAK-2 binding region in GHR molecule and was implicated in GH promotion of cell survival through inhibition of the pro-apoptotic caspase-3 [
41]. In addition, GH increases the expression of STAT-5b in colonic epithelial cells, which might be involved in the down-regulation of pro-apoptotic peroxisome proliferator activated receptor-γ expression [
42]. Besides, blocking endogenous GH action using a GHR antagonist has been associated with increased cell death [
6]. Recently, Keane et al. proposed that observed GH induced anti-apoptotic effects may in part be mediated also through a pathway that alters the concentration of particular mitochondrially-associated miRNAs that can control the expression of apoptosis-related genes [
43]. All the above mechanisms may simultaneously lead to observed GH-dependent anti-apoptotic effects in different types of cells, including CD34+ cells.
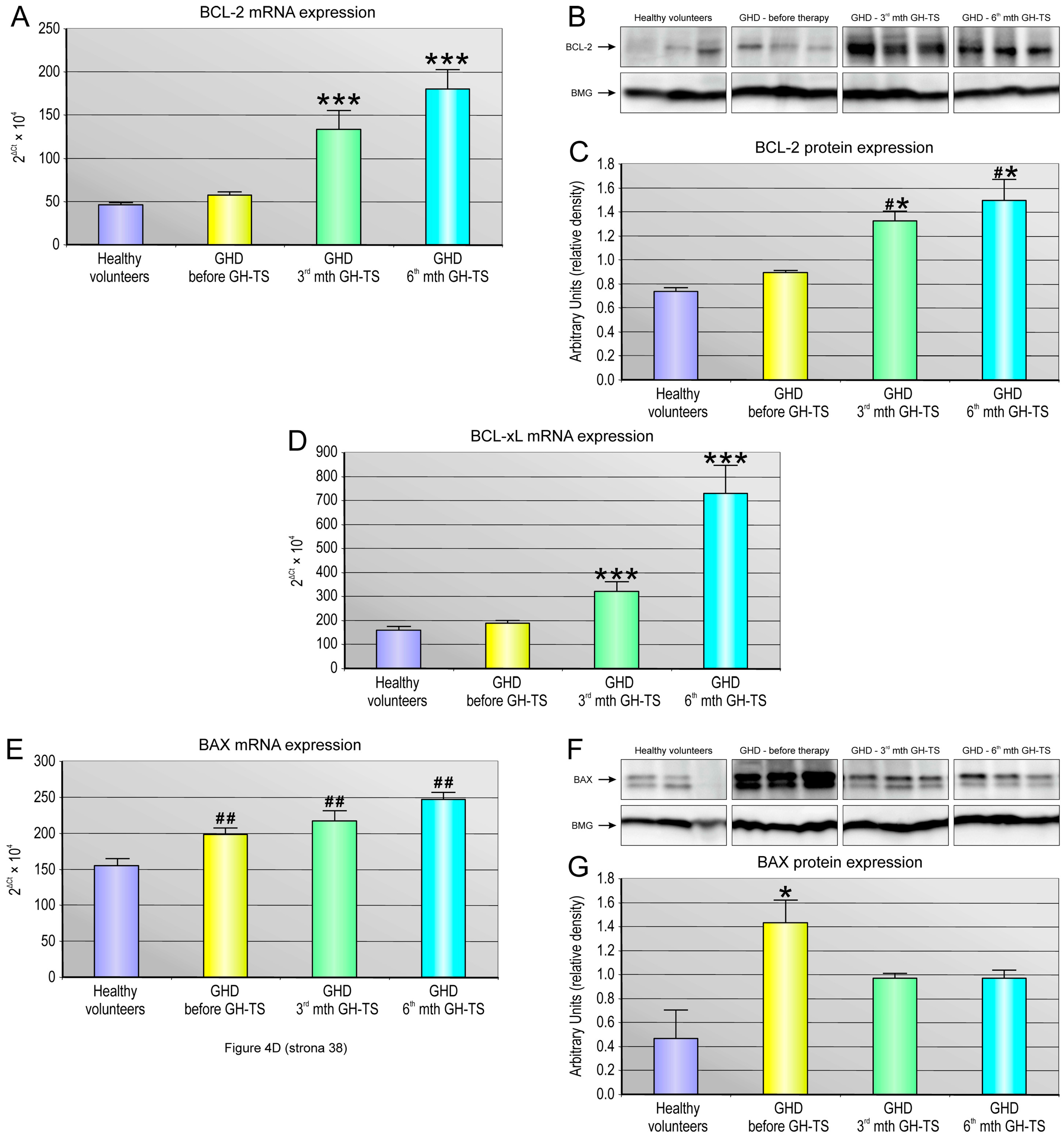
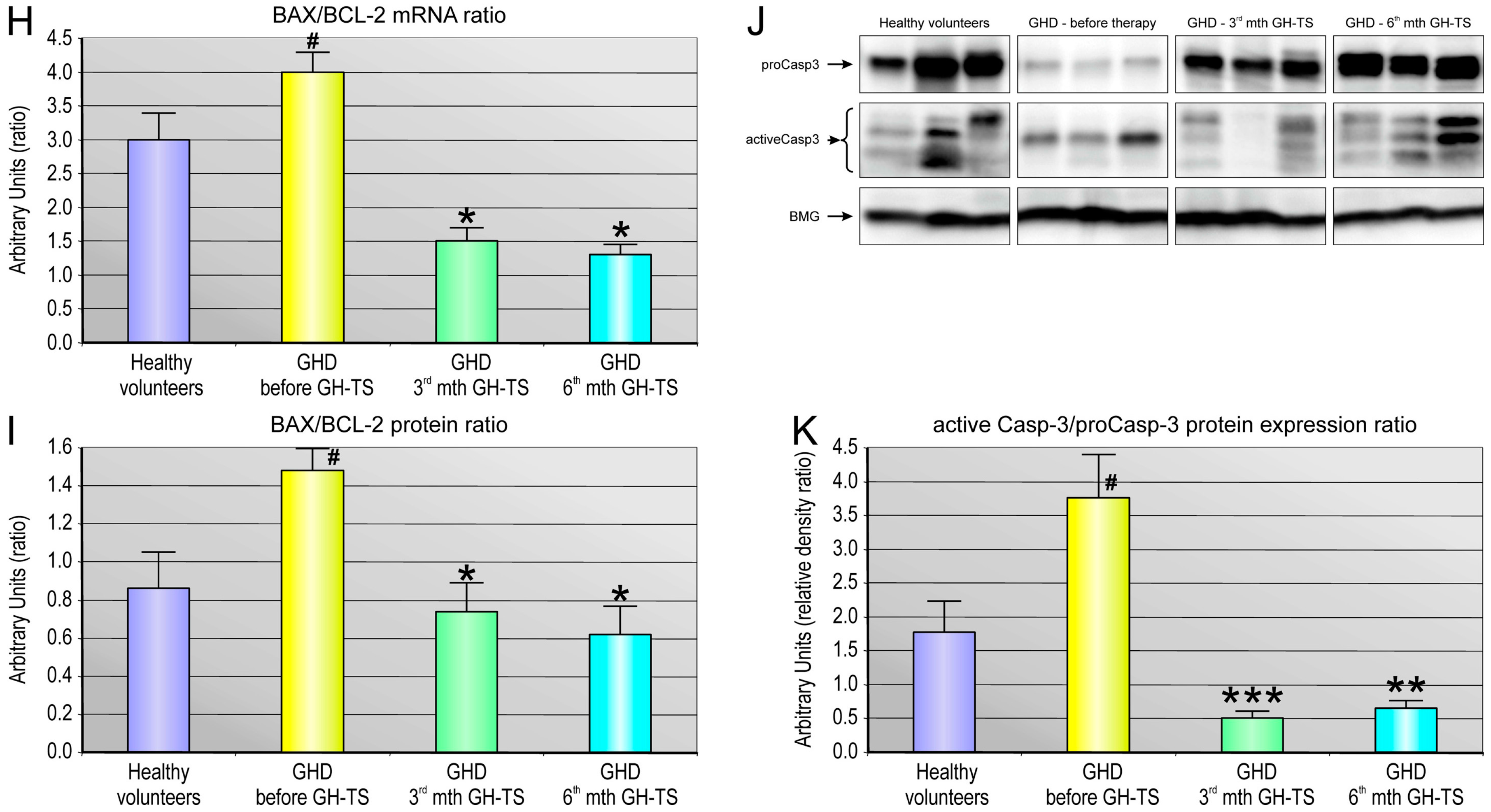
Apoptosis is regulated via the action of several oncogenes, and subsequently oncoproteins, which display inhibiting or promoting action. We demonstrated a strong relationship between the status of GH treatment and the expression of apoptosis-related genes such as anti-apoptotic BCL-2 and BCL-XL and the pro-apoptotic BAX gene. In particular, we investigated whether these genes with cell death/survival functions were differentially modulated by GH in hematopoietic CD34+ cells from GHD children. The BCL-2 gene encodes a protein that blocks programmed cell death without affecting cellular proliferation [
44]. We found that CD34+ cells harvested from GH-treated patients exhibited significantly increased expression of both BCL-2 and BCL-XL genes at all examined time points of GH therapy. Our data are consistent with those obtained in previous studies and indicate that GH delivery to hematopoietic cells has a positive impact on the expression of selected anti-apoptotic genes in these cells [
8,
9,
10]. The BAX protein is a member of the BCL-2 family that promotes apoptosis [
45]. Here, the CD34+ cells from GHD patients examined prior to the GH treatment exhibited significantly increased expression of the BAX gene transcripts, and its corresponding BAX protein, compared to their controls. In our study, we surprisingly found that BAX gene was also significantly up-regulated in CD34+ cells from GHD patients treated with GH. This up-regulation might be a result of more rapid cellular turnover as well as an increased need for unnecessary cell elimination throughout hematopoietic lineage cell development, and both processes could be initially stimulated by exogenous GH. Additionally, this result may suggest that GH replacement during the first 6 months might be insufficient to block the pro-apoptotic mechanisms induced in GHD patients presenting the chronic endogenous GH deficiency conditions. On the other hand, the opposite results were reported by a number of authors, who demonstrated that expression of BAX gene together with other pro-apoptotic factors, such as BAD or caspases, were down-regulated in an in vitro model of T cell lymphoma over-expressing GH [
12] and in cardiomyocytes [
46] or colonocytes [
47] of transgenic mice overexpressing GH gene as well as in myocytes [
48] and neurons [
49] of GH-treated animals. Unfortunately, at this stage of our research, it is impossible to define the exact mechanism of the observed BAX gene up-regulation during GH treatment. However, to evaluate whether GH treatment could potentially induce apoptosis via imbalance of BCL-2 and BAX expression in these cells, we performed quantitative analysis of BAX to BCL-2 ratio following GH treatment at mRNA and protein level. This ratio serves as a rheostat to determine the cell susceptibility to apoptosis, as increased BAX/BCL-2 ratio would favor cytochrome c release and activation of the mitochondrion-mediated signaling of apoptotic pathway with a final up-regulation of pro-apoptotic caspase-3 [
45]. Therefore, we analyzed the BAX/BCL-2 mRNA and BAX/BCL-2 protein ratio in CD34+ cells from all the groups, and we found that GH therapy is associated with the pronounced reduction in the BAX/BCL-2 ratio at mRNA and protein level in 3rd and 6th month of GH-TS. This observation is in agreement with the earlier report [
48] and with another finding of this study that GH therapy could significantly decrease the caspase-3/procaspase-3 ratio in cells from the GHD patients, as observed in both the 3rd and 6th month of GH treatment. Because caspase-3 exists as zymogen that must first be proteolytically cleaved to become activated protease, the caspase-3/procaspase-3 ratio has been used here as an index of caspase-3 activation in examined cells. In contrast, GHD patients examined prior to GH therapy demonstrated the increased BAX to BCL-2 ratio at both RNA and protein level compared to controls. Consequently, in untreated GHD patients, the apoptosis-associated caspase-3 activation was also significantly up-regulated.
Therefore, we decided to verify potential associations between systemic levels of GH and the expression of apoptosis-related genes in CD34+ cells from GHD patients comparing the genome-wide gene expression profiles of these cells collected from all the groups. We were interested in genes involved in such biological processes concerning induction and regulation of programmed cell death, extrinsic/intrinsic apoptotic signaling pathways, regulation of apoptotic signaling pathways, apoptosis-related nuclear and mitochondrial changes, cell-type specific apoptotic processes, execution phase of apoptosis and its regulation, regulation of enzyme activity involved in apoptotic process, regulation of hematopoietic cell apoptosis, and apoptotic signaling pathway in response to DNA damage. Comparison of the bioinformatics analyses of the complex gene datasets of examined cells identified that 6-month-GH treatment repressed significantly 25 pro-apoptotic genes and TNF was decreased the most. In parallel, the chronic GH administration significantly up-regulated only 8 pro-apoptotic genes. Since these genes are related not only to apoptosis induction, but also to pathways related to regulation of immune system processes and production of cytokines involved in the immune/stress responses of hematopoietic cells, the present study suggests that GH may play an inhibitory role on TNF-alpha production in human hematopoietic cells and therefore GH may also indirectly inhibit apoptosis by down-regulation of genes related to TNF-response signaling pathways. Our results seem to be in concert with the report of Andiran et al. [
50], who found that in children with GHD, the TNF-alpha levels in PB decreased significantly during GH treatment, demonstrating a reverse correlation between TNF-alpha and GH concentrations in PB. Likewise, Serri et al. [
51] examined the effect of GH deficiency and subsequent GH replacement on monocyte cytokine production and found that GH treatment of GHD patients led to a reduction in plasma TNF-alpha and IL-6 levels, and the decreased production of both cytokines from monocytes in vitro. Other studies showed that GH administration reduces circulating soluble apoptosis mediators, including TNF system components [
52,
53]. Altogether, our data suggest that GH may play a role in modulating the TNF-alpha expression in CD34+ cells. In contrast, among the anti-apoptotic/pro-survival genes with the largest expression change during GH therapy, all detected genes were up-regulated (14 genes), and our data suggest that GH is capable of effectively stimulate the anti-apoptotic signaling pathways. Interestingly, the most up-regulated gene in this group (almost 6 folds) was gene of cyclin-dependent kinase-6 (CDK6), which encodes serine/threonine-protein kinase involved in the control of the entrance into the cell cycle via interacting with D-type cyclins during G1 interphase and promotes G1/S transition in the cell cycle [
54]. Concurrently, we found that CDK6-related cyclin D2 (CCND2) was also significantly up-regulated in CD34+ cells by GH therapy. Of note, CDK6 kinase is also implicated in initiation and maintenance of cell cycle exit during cell differentiation and is required for the proliferation of specific hematopoietic cell types (e.g., erythroid cells) [
55], and it was reported that the TNF-alpha down-regulates CDK6 expression and induces apoptosis in human immature erythroid BFU-E cells [
56]. In this notion, as GH administration significantly decreases TNF-alpha production, it would derepress CDK6 signaling pathway and this might be possible explanation how GH induces cell cycle and reversely inhibits apoptosis in hematopoietic cells. In addition, we found using microarray method the increased expression of several members of the BCL-2 gene family, such as BCL2A1 and BCL3, and these results corroborated our quantitative PCR data. This finding is also consistent with previous results obtained using human monocytic cells, promyelocytic leukemia U937 cell line or other immune-related cells [
8,
57]. Altogether, the increased gene expression of anti-apoptotic molecules in course of recombinant human GH therapy could be attributed to the activation of the body’s defense mechanisms to fight against the increased apoptosis rate, while insufficient amount of endogenous GH is present in GHD children prior to GH therapy. Especially, these data provide evidence that GH mediates its protective effect through enhancing the expression of the anti-apoptotic oncoprotein BCL-2 and other members of BCL-2-dependent pathways.
4. Materials and Methods
4.1. Subjects
We enrolled 40 children with height lower than the 3rd percentile on a growth chart, who were diagnosed with severe isolated GHD according to the established clinical criteria [
58]. The GH-TS was performed by daily subcutaneous GH injections at 0.031 mg/kg/d. None of the patients suffered from diabetes insipidus, chromosomal abnormalities, dysmorphic syndromes, intestinal malabsorption, other chronic diseases or acquired GHD, as confirmed by a full clinical and laboratory evaluation. All subjects exhibited normal thyroid and adrenal function. Magnetic resonance imaging of hypothalamus and pituitary region was normal in all patients. 60 healthy children of similar ages, who did not differ significantly from GHD patients in terms of puberty and bone age, constituted the control group. The children’s pubertal stages were rated according to the Tanner method and are summarized in
Table 1. All procedures were approved by the Local Ethics Committee of the Pomeranian Medical University and informed consent was provided for each patient.
4.2. Laboratory Measurements and Cell Isolation
PB samples were collected at the moment of GHD diagnosis and after 3 and 6 months of GH-TS and we determined hematological and hormonal parameters and isolated CD34+ cells. The selected hematological parameters were evaluated using cell analyzer (Cell Dyn 3000, Abbott Diagnostics, Mountain View, CA, USA). The mononuclear cell fraction was isolated by density gradient centrifugation and depleted of adherent and T cells. This fraction was next enriched for CD34+ cells using the CD34 MicroBead Kit (Miltenyi Biotech Inc., Auburn, CA, USA) according to the manufacturer’s protocol. Isolated CD34+ cells were next analyzed by flow cytometry to evaluate the efficiency of the CD34-positive sorting. CD34 antigen was expressed in 91.2% ± 4.8% of immunomagnetically isolated cells. The mean number of CD34+ cells collected from one subject during isolation procedure was 150,000 ± 105,000 cells in average.
4.3. Immunofluorescence Staining of PB-Derived CD34+ Cells
The CD34+ cells population was subjected to immunofluorescence staining for GHR. Briefly, isolated cells were fixed in 4% paraformaldehyde for 20 min and washed in PBS. GHR protein was detected using monoclonal PE-conjugated mouse anti-human GHR antibody (clone: MAB-1, Santa Cruz Biotechnology, Santa Cruz, CA, USA). CD45 protein was detected using FITC-conjugated anti-human monoclonal IgG (BD Biosciences, San Jose, CA, USA). Cells were subsequently labeled with DAPI (BD Biosciences) for nuclear staining. Fluorescent images were captured using Pathway Bioimager System (BD Biosciences).
4.4. Flow Cytometry
The CD34+ cells were quantitatively analyzed for the expression of GHR using flow cytometry. In particular, the mean fluorescence intensity (MFI) of GHR staining was assessed. Briefly, CD34+ cells were incubated (30 min) with mouse anti-human fluorochrome-conjugated monoclonal antibodies against specific antigens, including GHR, CD34, and CD45 (all from BD Biosciences). The cells were washed twice in ice-cold PBS, resuspended in 1% paraformaldehyde and analyzed in flow cytometer (LSRII, BD Biosciences) using the BD FACSDiva software.
4.5. Apoptosis Detection
The level of spontaneous apoptosis in CD34+ cells circulating in PB of GHD patients and controls was measured using two different methods. The Annexin V-FITC Apoptosis Detection Kit II (BD Biosciences) was used for the detection of early stage of apoptosis. To detect the late stage of apoptosis, the collected CD34+ cells were analyzed with the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using the APO-Direct Kit (BD Biosciences). Both kits were used according to the manufacturer’s instructions.
4.6. RNA Isolation and Gene Expression Analysis
Total mRNA was isolated from CD34+ cells using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Subsequently, mRNA was reverse transcribed using the First Strand cDNA Synthesis Kit (Fermentas International Inc., Burlington, ON, Canada). Quantitative assessment of BCL-2, BCL-XL, BAX mRNA levels was performed using real time QRT-PCR carried out on a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad Inc., Hercules, CA, USA). The 25-µL reaction mixture contained 12.5 µL of SYBR Green PCR Master Mix, 10 ng of cDNA template, and one pair of the primers listed in
Table 6. The relative quantification value of the target gene was normalized to the endogenous control gene of beta-2 microglobulin (BMG) and expressed as 2Δ
Ct, where Δ
Ct = [
Ct of BMG] − [
Ct of target gene].
4.7. RNA Microarray Data Analysis
Total RNA was isolated from CD34+ cells using RNeasy Mini Kit (Qiagen). RNA was isolated from CD34+ cells of five GH-treated patients at baseline, and in 3rd and 6th month of treatment, and of five control subjects, and next was pooled to generate the final RNA sample representing a particular group of patients. Sense-strand cDNA generated from total RNA using an Ambion WT Expression Kit (Life Technologies, Paisley, UK) was fragmented and labeled using the GeneChipH WT Terminal Labeling Kit (Affymetrix, Santa Clara, CA, USA) and hybridized onto an Affymetrix WT Array Strip. Hybridization as well as subsequent fluidics and scanning steps were performed using an Affymetrix GeneAtlasTM system (Affymetrix). Differences in the expression of the chosen genes and Gene Ontology (GO) terms were analyzed in the R programming environment using Bioconductor packages.
4.8. ELISA
The systemic levels of IGF-1 were measured using commercially available, high-sensitivity ELISA Quantikine human immunoassay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
4.9. Western Blot Analysis
Total protein was isolated from CD34+ cells using PARIS kit (Life Technologies, Paisley, UK). Protein was isolated from CD34+ cells of seven GH-treated patients at baseline, and in 3rd and 6th month of treatment, and of seven control subjects, and next was pooled to generate the final protein sample representing a particular group of patients. Briefly, cells were homogenized in the Cell Disruption Buffer containing protease and phosphatase inhibitors (10 µg/mL leupeptin, 10 µg/mL aprotinin, 1 µg/mL pepstatin A, 1 mM sodium fluoride, and 2 mM Na3VO4) (all from Sigma Aldrich, St. Louis, MO, USA). The mixture was centrifuged, supernatants were collected, and protein concentrations were determined using the Bradford protein assay (Sigma Aldrich). Equal amounts of protein (20 µg/well) were loaded and separated by 4%–20% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, mini-PROTEAN II electrophoresis system, Bio-Rad, Hercules, CA, USA) and then transferred to a 0.2-µm polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). The membrane was probed with selected primary monoclonal/polyclonal IgG antibodies directed against the amino acid sequences of BCL-2, BAX, cleaved caspase-3, procaspase-3 and phosphorylated STAT-5 (all from Cell Signaling Technology, Beverly, MA, USA). Immunoreactive bands were detected using horseradish peroxidase-conjugated goat anti-rabbit and donkey-anti-goat secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Chemiluminescence detection was performed using the ECL Select Detection Kit (GE Healthcare, formerly Amersham Life Sciences, Little Chalfont, UK), and bands were subsequently visualized using a UVP camera (Gel DOC-It Imaging system; Bio-Rad, Hercules, CA, USA). Loading in the lanes was evaluated by stripping the blots for 2 h at 37 °C and then overnight at room temperature (IgG Elution Buffer; Thermo Fisher Scientific, Waltham, MA, USA). Reprobing was then performed in an analogous manner with a human anti-BMG monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:1000 dilution, followed by an HRP-conjugated secondary antibody as described above. Protein levels were analyzed densitometrically by ImageJ software. Re-probing blots with more than one antibody against phosphorylated and total protein was performed in the same PVDF membranes. Each group analyzed in WB was performed in triplicate of biological replication.
4.10. Statistical Methods
Differences in the values of the quantitative parameters were compared between groups by unpaired Student t-test with Welch’s correction; for non-parametric tests, values were compared using the Mann-Whitney test. A p value of <0.05 was considered statistically significant.