A Mixture of Persistent Organic Pollutants and Perfluorooctanesulfonic Acid Induces Similar Behavioural Responses, but Different Gene Expression Profiles in Zebrafish Larvae
Abstract
:1. Introduction
2. Results
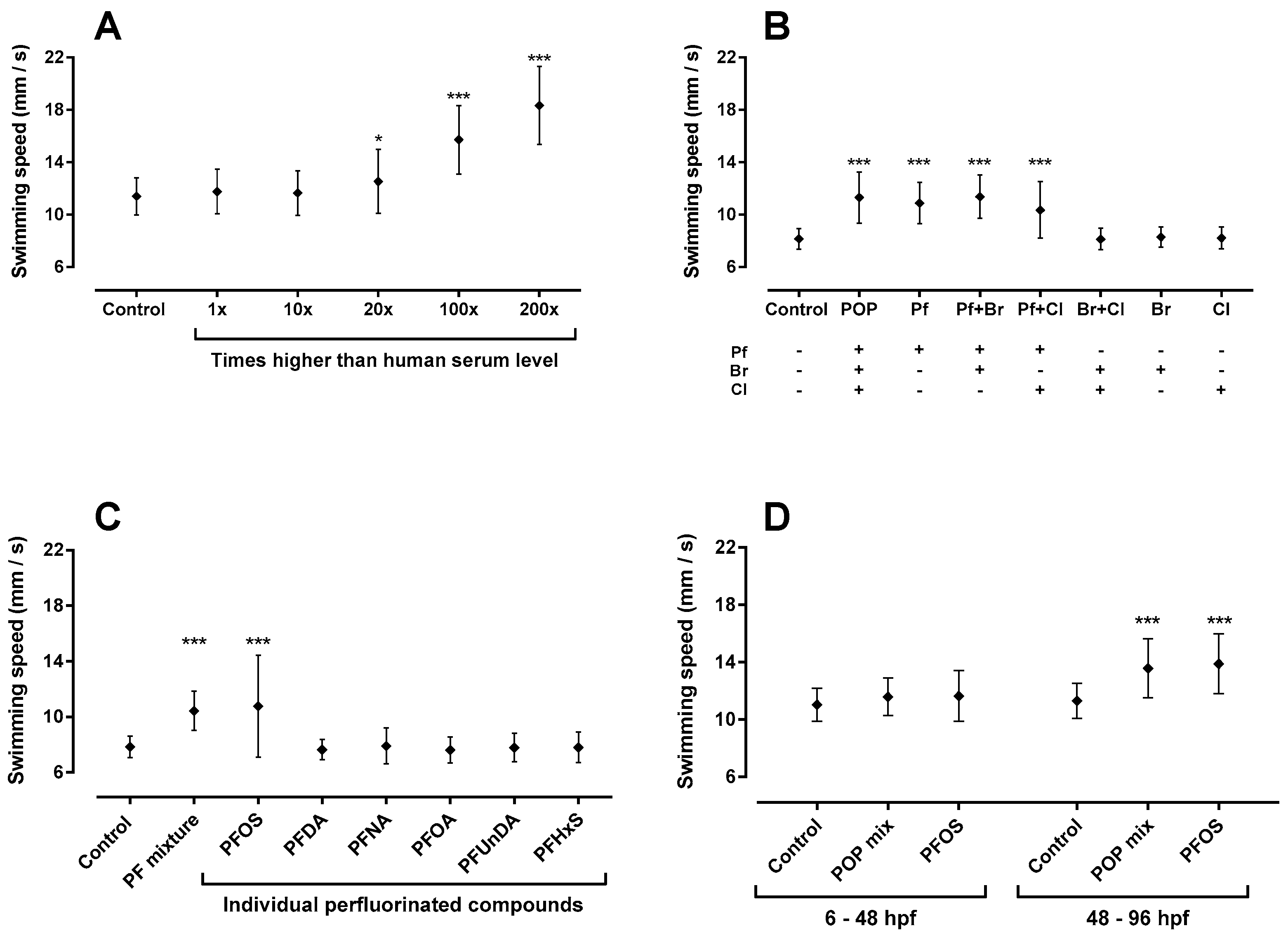
2.1. Total Persistent Organic Pollutant (POP) Mixture Increased Swimming Speed
2.2. Sub-Mixtures Containing Perfluorinated Compounds Increased Swimming Speed
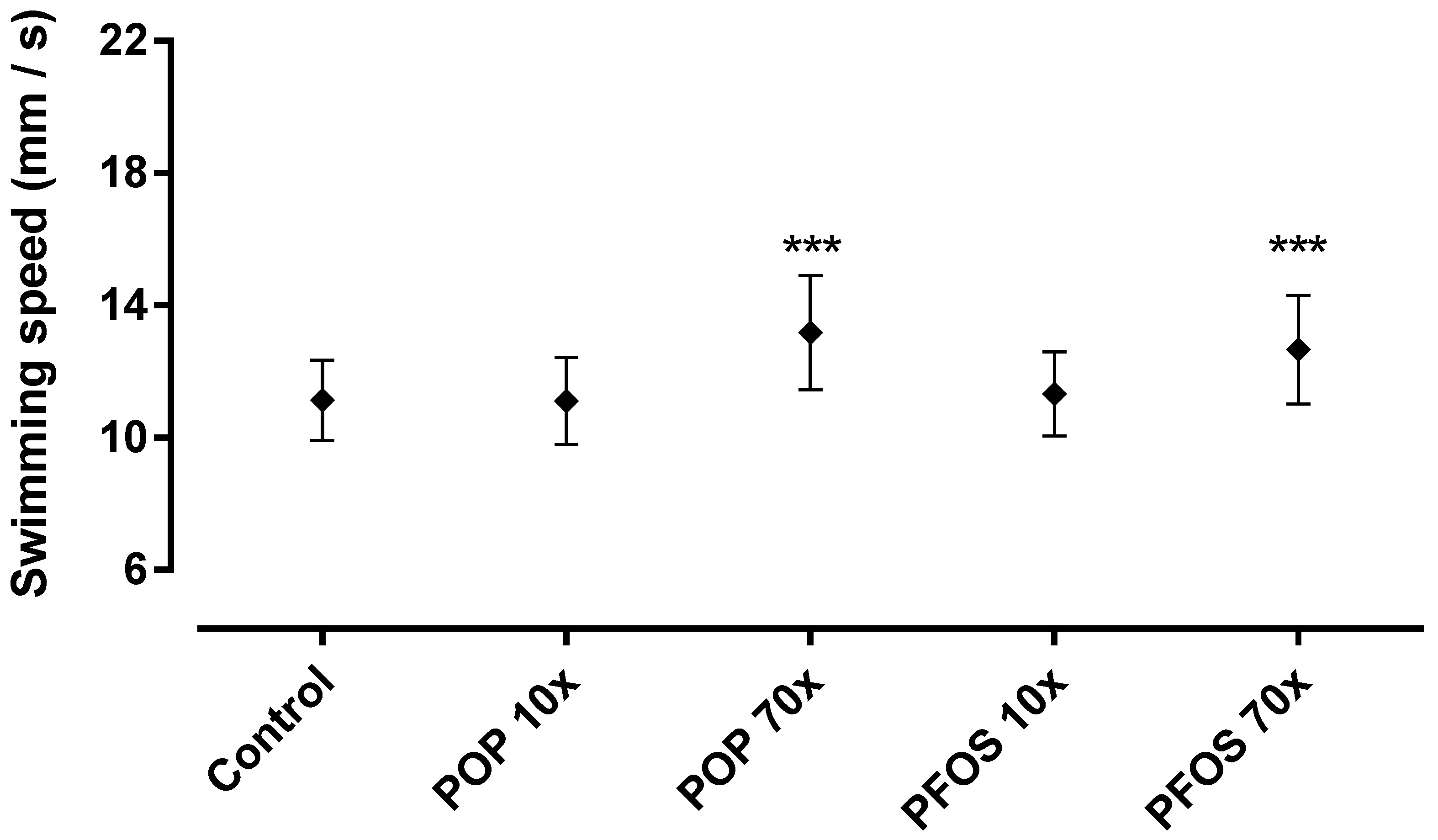
2.3. Perfluorooctanesulfonic Acid (PFOS) Increased Swimming Speed
2.4. PFOS Tissue Uptake in Larvae
2.5. 48–96 hpf as Developmental Window of Sensitivity
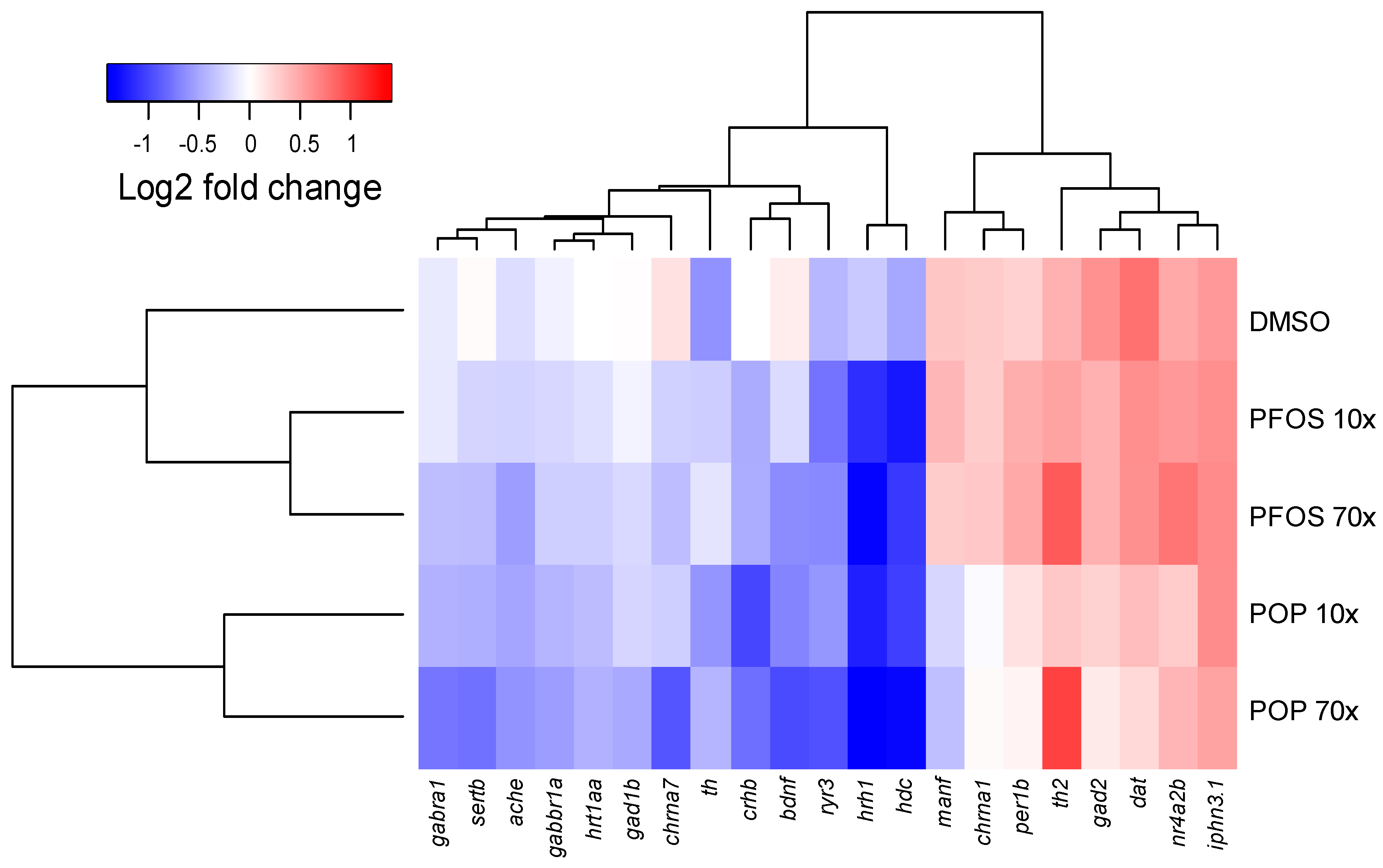
2.6. POP Mixture and PFOS Altered Gene Expression Differently
3. Discussion
4. Materials and Methods
4.1. Mixtures and Chemicals
4.2. Zebrafish Maintenance and Breeding
4.3. Exposure Scenario
4.4. Locomotor Activity
4.5. PFOS Tissue Uptake in Larvae
4.6. Developmental Sensitivity Test
4.7. Gene Transcription Analysis
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BDE | Brominated diphenyl ethers |
| BDNF | Brain-derived neurotrophic factor |
| CHRNA7 | Cholinergic receptor nicotinic alpha 7 subunit |
| CNS | Central nervous system |
| CRHB | Corticotropin releasing hormone Beta |
| DMSO | Dimethyl sulfoxide |
| DPF | Day post fertilization |
| GABRA1 | Gamma-Aminobutyric Acid Type A Receptor Alpha1 Subunit |
| HDC | Histidine decarboxylase |
| HPF | Hour post fertilization |
| HRH1 | Histamine Receptor H1 |
| LME | Linear mixed effect |
| MANF | Mesencephalic astrocyte-derived neurotrophic factor |
| PBDE | Polybrominated diphenyl ethers |
| PCB | Polychlorinated biphenyl |
| PFDA | Perfluorodecanoic acid |
| PFHxS | Perfluorohexane sulfonate |
| PFNA | Perfluorononanoic acid |
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctanesulfonic acid |
| PFUnDA | Perfluoroundecanoic acid |
| POPS | Persistent organic pollutants |
| ROS | Reactive oxygen species |
| SERTB | Serotonin transporter B |
Appendix A
| Exposure Groups | Distance Moved (mm/10 min) | Swimming Time (s/10 min) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1× | 10× | 20× | 100× | 200× | Control | 1× | 10× | 20× | 100× | 200× | |
| Total POPs | 742.1 ± 290.2 | 782.5 ± 373.4 | 735.9 ± 303.9 | 783.2 ± 271.7 | 722.1 ± 366.2 | 596.3 ± 400.8 * | 66.1 ± 27.1 | 67.2 ± 31.9 | 64.1 ± 25.3 | 64.6 ± 23.8 | 48.3 ± 26.8 ** | 34 ± 24.3 *** |
| Pf | 730.8 ± 345.3 | 730.5 ± 371.7 | 774.4 ± 385.3 | 722.8 ± 370 | 743.6 ± 306.7 | 552.4 ± 367.4 * | 61.7 ± 29.8 | 61 ± 32.3 | 66.2 ± 34.6 | 60.2 ± 31.7 | 47.6 ± 23.9 * | 32.6 ± 24.1 *** |
| Pf + Br | 784.8 ± 420.4 | 720.4 ± 326.2 | 672.8 ± 368.9 | 834.1 ± 331.1 | 657.2 ± 330.9 | 457.2 ± 321 *** | 66.9 ± 35.3 | 63.3 ± 29.3 | 55.7 ± 31.3 | 69.2 ± 28.4 | 42.9 ± 24 *** | 25.4 ± 18.5 *** |
| Pf + Cl | 667.4 ± 330.8 | 689.4 ± 305.7 | 675.9 ± 276.3 | 669.2 ± 313.3 | 632.4 ± 369.2 | 494.5 ± 313.8 * | 57.8 ± 27.3 | 59 ± 27 | 57.3 ± 24.7 | 54.9 ± 24.4 | 42 ± 28.7 * | 30.7 ± 23.9 *** |
| Br | 622.5 ± 309.6 | 562.6 ± 238.3 | 614.9 ± 283.4 | 622.8 ± 293.6 | 656.8 ± 238.3 | 679.6 ± 297.2 | 60.3 ± 30.1 | 52.4 ± 23.2 | 56.9 ± 27.3 | 56.7 ± 27.2 | 59.9 ± 23.4 | 62.6 ± 27.8 |
| Cl | 770.6 ± 341.4 | 684.1 ± 310.5 | 711.9 ± 269.8 | 738.1 ± 330.8 | 889.7 ± 374.6 | 838.8 ± 403.6 | 68.4 ± 30.1 | 58 ± 27.2 | 60.5 ± 24.4 | 64.1 ± 29.8 | 77 ± 32.6 | 71.3 ± 33.7 |
| Br + Cl | 677.7 ± 338.2 | 627.4 ± 319.9 | 641.7 ± 342.3 | 654.4 ± 329.8 | 640 ± 327.3 | 706.4 ± 277.5 | 62.6 ± 31.9 | 58.4 ± 31.4 | 58.1 ± 32.8 | 58.4 ± 29.4 | 57.7 ± 30.6 | 64.2 ± 26.9 |
Appendix B

Appendix C
References
- Hung, H.; Katsoyiannis, A.A.; Guardans, R. Ten years of global monitoring under the stockholm convention on persistent organic pollutants (POPs): Trends, sources and transport modelling. Environ. Pollut. 2016, 217, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Daley, J.M.; Paterson, G.; Drouillard, K.G. Bioamplification as a bioaccumulation mechanism for persistent organic pollutants (POPs) in wildlife. Rev. Environ. Contam. Toxicol. 2014, 227, 107–155. [Google Scholar] [PubMed]
- Kim, S.; Park, J.; Kim, H.J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.B.; Kim, S.; et al. Association between several persistent organic pollutants and thyroid hormone levels in serum among the pregnant women of korea. Environ. Int. 2013, 59, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Kvalem, H.E.; Thomsen, C.; Frøshaug, M.; Haugen, M.; Becher, G.; Alexander, J.; Meltzer, H.M. Dietary exposure to brominated flame retardants correlates with male blood levels in a selected group of norwegians with a wide range of seafood consumption. Mol. Nutr. Food Res. 2008, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Linderholm, L.; Biague, A.; Mansson, F.; Norrgren, H.; Bergman, A.; Jakobsson, K. Human exposure to persistent organic pollutants in west Africa—A temporal trend study from guinea-bissau. Environ. Int. 2010, 36, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Polder, A.; Thomsen, C.; Lindström, G.; Løken, K.B.; Skaare, J.U. Levels and temporal trends of chlorinated pesticides, polychlorinated biphenyls and brominated flame retardants in individual human breast milk samples from northern and southern norway. Chemosphere 2008, 73, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Pumarega, J.; Gasull, M.; Lee, D.H.; Lopez, T.; Porta, M. Number of persistent organic pollutants detected at high concentrations in blood samples of the united states population. PLoS ONE 2016, 11, e0160432. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, E.; Grimalt, J.O.; Fernandez-Somoano, A.; Tardon, A. Transport of persistent organic pollutants across the human placenta. Environ. Int. 2014, 65, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; de Laat, R.; Tagliaferri, S.; Pellacani, C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett. 2014, 230, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Costa, L.G. Developmental neurotoxicity: Some old and new issues. ISRN Toxicol. 2012, 2012, 814795. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, P.R.S. Neurotoxicity of persistent organic pollutants: Possible mode(s) of action and further considerations. Dose Response 2005, 3, 273–305. [Google Scholar] [CrossRef] [PubMed]
- Berghuis, S.A.; Bos, A.F.; Sauer, P.J.; Roze, E. Developmental neurotoxicity of persistent organic pollutants: An update on childhood outcome. Arch. Toxicol. 2015, 89, 687–709. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108 (Suppl. 3), 511–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, B.; Zhao, W.D.; Liu, Y.J.; Shang, D.S.; Fang, W.G.; Chen, Y.H. Perfluorooctane sulfonate triggers tight junction “opening” in brain endothelial cells via phosphatidylinositol 3-kinase. Biochem. Biophys. Res. Commun. 2011, 410, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Das, S.R.; La Du, J.; Corvi, M.M.; Bai, C.; Chen, Y.; Liu, X.; Zhu, G.; Tanguay, R.L.; Dong, Q.; et al. Chronic PFOS exposures induce life stage-specific behavioral deficits in adult zebrafish and produce malformation and behavioral deficits in F1 offspring. Environ. Toxicol. Chem. 2013, 32, 201–206. [Google Scholar] [CrossRef]
- Hallgren, S.; Fredriksson, A.; Viberg, H. More signs of neurotoxicity of surfactants and flame retardants—Neonatal PFOS and PBDE 99 cause transcriptional alterations in cholinergic genes in the mouse CNS. Environ. Toxicol. Pharmacol. 2015, 40, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, C.; Wang, L.; Ye, X.; Bai, C.; Simonich, M.T.; Tanguay, R.L.; Dong, Q. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat. Toxicol. 2010, 98, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Lee, Y.J.; Yang, J.H. Perfluorooctane sulfonate induces apoptosis of cerebellar granule cells via a ROS-dependent protein kinase C signaling pathway. Neurotoxicology 2012, 33, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Lopez-Doval, S.; Pereiro, N.; Lafuente, A. Perfluorooctane sulfonate (PFOS) exposure could modify the dopaminergic system in several limbic brain regions. Toxicol. Lett. 2016, 240, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, L.; Sun, W.; Xue, Z. Effects of perfluorooctane sulfonate on amino acid neurotransmitters and glutamine synthetase in rats. J. Hyg. Res. 2009, 38, 19–21. [Google Scholar]
- Shi, X.; Zhou, B. The role of Nrf2 and mapk pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol. Sci. 2010, 115, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Ge, X.; Wang, D.; Wang, T.; Zhang, L.; Tanguay, R.L.; Simonich, M.; Huang, C.; Dong, Q. Chronic perfluorooctanesulphonic acid (PFOS) exposure produces estrogenic effects in zebrafish. Environ. Pollut. 2016, 218, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tanguay, R.L.; Tal, T.L.; Gai, Z.; Ma, X.; Bai, C.; Tilton, S.C.; Jin, D.; Yang, D.; Huang, C.; et al. Early life perfluorooctanesulphonic acid (PFOS) exposure impairs zebrafish organogenesis. Aquat. Toxicol. 2014, 150, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Spulber, S.; Kilian, P.; Wan Ibrahim, W.N.; Onishchenko, N.; Ulhaq, M.; Norrgren, L.; Negri, S.; Di Tuccio, M.; Ceccatelli, S. PFOS induces behavioral alterations, including spontaneous hyperactivity that is corrected by dexamfetamine in zebrafish larvae. PLoS ONE 2014, 9, e94227. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Oliveri, A.; Levin, E.D. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 14–23. [Google Scholar] [CrossRef] [PubMed]
- De Esch, C.; Slieker, R.; Wolterbeek, A.; Woutersen, R.; de Groot, D. Zebrafish as potential model for developmental neurotoxicity testing: A mini review. Neurotoxicol. Teratol. 2012, 34, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Linney, E.; Upchurch, L.; Donerly, S. Zebrafish as a neurotoxicological model. Neurotoxicol. Teratol. 2004, 26, 709–718. [Google Scholar] [CrossRef]
- Melvin, S.D.; Wilson, S.P. The utility of behavioral studies for aquatic toxicology testing: A meta-analysis. Chemosphere 2013, 93, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Rihel, J.; Schier, A.F. Behavioral screening for neuroactive drugs in zebrafish. Dev. Neurobiol. 2012, 72, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Tierney, K.B. Behavioural assessments of neurotoxic effects and neurodegeneration in zebrafish. Biochim. Biophys. Acta 2011, 1812, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Sloman, K.A. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 2004, 68, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, C.; Hu, C.; Yu, K.; Yang, L.; Zhou, B. Acute exposure to DE-71: Effects on locomotor behavior and developmental neurotoxicity in zebrafish larvae. Environ. Toxicol. Chem. 2012, 31, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Puttonen, H.; Sundvik, M.; Rozov, S.; Chen, Y.-C.; Panula, P. Acute ethanol treatment upregulates Th1, Th2, and hdc in larval zebrafish in stable networks. Front. Neural Circuits 2013, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Wu, Y.; Huang, C.; Wang, Q.; Han, J.; Guo, Y.; Shi, X.; Zhou, B. The developmental neurotoxicity of pbdes: Effect of DE-71 on dopamine in zebrafish larvae. Environ. Toxicol. Chem. 2015, 34, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, C.; Wang, X.; Chen, J.; Bai, C.; Chen, Y.; Chen, X.; Dong, Q.; Yang, D. BDE-47 disrupts axonal growth and motor behavior in developing zebrafish. Aquat. Toxicol. 2012, 120–121, 35–44. [Google Scholar] [CrossRef]
- National Research Council. Complex Mixtures: Methods for In Vivo Toxicity Testing; National Academies Press: Washington, DC, USA, 1988. [Google Scholar]
- Daouk, T.; Larcher, T.; Roupsard, F.; Lyphout, L.; Rigaud, C.; Ledevin, M.; Loizeau, V.; Cousin, X. Long-term food-exposure of zebrafish to PCB mixtures mimicking some environmental situations induces ovary pathology and impairs reproduction ability. Aquat. Toxicol. 2011, 105, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Scholze, M.; Ferreira, A.M.; Martins, M.; Correia, A.D. The joint effect of polycyclic aromatic hydrocarbons on fish behavior. Environ. Res. 2008, 108, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Péan, S.; Daouk, T.; Vignet, C.; Lyphout, L.; Leguay, D.; Loizeau, V.; Bégout, M.-L.; Cousin, X. Long-term dietary-exposure to non-coplanar pcbs induces behavioral disruptions in adult zebrafish and their offspring. Neurotoxicol. Teratol. 2013, 39, 45–56. [Google Scholar] [CrossRef]
- Keiter, S.; Baumann, L.; Färber, H.; Holbech, H.; Skutlarek, D.; Engwall, M.; Braunbeck, T. Long-term effects of a binary mixture of perfluorooctane sulfonate (PFOS) and bisphenol a (BPA) in zebrafish (Danio rerio). Aquat. Toxicol. 2012, 118–119, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, S.; You, H.; Liu, Z. Effects of zno nanoparticles on perfluorooctane sulfonate induced thyroid-disrupting on zebrafish larvae. J. Environ. Sci. 2016, 47, 153–164. [Google Scholar] [CrossRef]
- Wilson, J.; Berntsen, H.F.; Zimmer, K.E.; Frizzell, C.; Verhaegen, S.; Ropstad, E.; Connolly, L. Effects of defined mixtures of persistent organic pollutants (POPs) on multiple cellular responses in the human hepatocarcinoma cell line, HepG2, using high content analysis screening. Toxicol. Appl. Pharmacol. 2016, 294, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Berntsen, H.F.; Zimmer, K.E.; Verhaegen, S.; Frizzell, C.; Ropstad, E.; Connolly, L. Do persistent organic pollutants interact with the stress response? Individual compounds, and their mixtures, interaction with the glucocorticoid receptor. Toxicol. Lett. 2016, 241, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Groten, J.P.; Feron, V.J.; Sühnel, J. Toxicology of simple and complex mixtures. Trends Pharmacol. Sci. 2001, 22, 316–322. [Google Scholar] [CrossRef]
- Onishchenko, N.; Fischer, C.; Wan Ibrahim, W.N.; Negri, S.; Spulber, S.; Cottica, D.; Ceccatelli, S. Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotoxic. Res. 2011, 19, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Colomina, M.T.; Vicens, P.; Domingo, J.L. Influence of maternal restraint stress on the long-lasting effects induced by prenatal exposure to perfluorooctane sulfonate (PFOS) in mice. Toxicol. Lett. 2007, 171, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hagenaars, A.; Stinckens, E.; Vergauwen, L.; Bervoets, L.; Knapen, D. Pfos affects posterior swim bladder chamber inflation and swimming performance of zebrafish larvae. Aquat. Toxicol. 2014, 157, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Abdolrahman, K.; Juan, G.H.J.; Anna, M.L.-S.; Theodore, H.; Erik, R. Toxin induced behavioural aberrations in larval zebrafish are dependent on minor methodological alterations: The importance of standardisation. Toxicol. Lett. submitted for publication. 2016. [Google Scholar]
- Guo, S. Using zebrafish to assess the impact of drugs on neural development and function. Expert Opin. Drug Discov. 2009, 4, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Murakami, S.; Ashikawa, Y.; Sasagawa, S.; Umemoto, N.; Shimada, Y.; Tanaka, T. Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anom. 2014, 55, 1–16. [Google Scholar] [CrossRef]
- Staal, Y.C.M.; Pushparajah, D.S.; van Herwijnen, M.H.M.; Gottschalk, R.W.H.; Maas, L.M.; Ioannides, C.; van Schooten, F.J.; van Delft, J.H.M. Interactions between polycyclic aromatic hydrocarbons in binary mixtures: Effects on gene expression and DNA adduct formation in precision-cut rat liver slices. Mutagenesis 2008, 23, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Doval, S.; Salgado, R.; Fernandez-Perez, B.; Lafuente, A. Possible role of serotonin and neuropeptide Y on the disruption of the reproductive axis activity by perfluorooctane sulfonate. Toxicol. Lett. 2015, 233, 138–147. [Google Scholar] [CrossRef]
- Shi, X.; Liu, C.; Wu, G.; Zhou, B. Waterborne exposure to PFOS causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Chemosphere 2009, 77, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N. Serotonergic control of aggressive behavior: Novel approaches—New interpretations. Zhurnal Vysshei Nervnoi Deiatelnosti Imeni I P Pavlova 2015, 65, 546–563. [Google Scholar] [PubMed]
- Tritsch, N.X.; Granger, A.J.; Sabatini, B.L. Mechanisms and functions of gaba co-release. Nat. Rev. Neurosci. 2016, 17, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sundvik, M.; Rozov, S.; Priyadarshini, M.; Panula, P. MANF regulates dopaminergic neuron development in larval zebrafish. Dev. Biol. 2012, 370, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Y.; Cheng, L.; Liu, B.; Zhang, W.; Guo, Y.J.; Nie, L. Mesencephalic astrocyte-derived neurotrophic factor inhibits oxygen-glucose deprivation-induced cell damage and inflammation by suppressing endoplasmic reticulum stress in rat primary astrocytes. J. Mol. Neurosci. 2013, 51, 671–678. [Google Scholar] [CrossRef] [PubMed]
- De Groef, B.; van der Geyten, S.; Darras, V.M.; Kuhn, E.R. Role of corticotropin-releasing hormone as a thyrotropin-releasing factor in non-mammalian vertebrates. Gen. Comp. Endocrinol. 2006, 146, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, Y.; Hirasawa, N. The role of histamine H1 and H4 receptors in atopic dermatitis: From basic research to clinical study. Allergol. Int. 2014, 63, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; Haggard, D.E.; Gonnerman, G.D.; Tanguay, R.L. Advanced morphological—Behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 2015, 145, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, T.; Yin, D.Q. Locomotor activity changes on zebrafish larvae with different 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) embryonic exposure modes. Chemosphere 2014, 94, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Lin, J.; Zhu, Y.; Liu, X.; Zhang, Y.; Ji, Y.; Yang, X.; Zhang, Y.; Guo, N.; Li, Q. Anxiety-related behavioral responses of pentylenetetrazole-treated zebrafish larvae to light-dark transitions. Pharmacol. Biochem. Behav. 2016, 145, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Smith, D. Worldwide trends in DDT levels in human breast milk. Int. J. Epidemiol. 1999, 28, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, H.F.; Vidar, B.; Cathrine, T.; Erik, R.; Karin, E.Z. The synthesis of an environmentally relevant mixture of persistent organic pollutants for use in in vivo and in vitro studies. Toxicol. Lett. submitted for publication. 2016. [Google Scholar]
- Padilla, S.; Hunter, D.L.; Padnos, B.; Frady, S.; MacPhail, R.C. Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicol. Teratol. 2011, 33, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Bytingsvik, J.; van Leeuwen, S.P.; Hamers, T.; Swart, K.; Aars, J.; Lie, E.; Nilsen, E.M.; Wiig, O.; Derocher, A.E.; Jenssen, B.M. Perfluoroalkyl substances in polar bear mother-cub pairs: A comparative study based on plasma levels from 1998 and 2008. Environ. Int. 2012, 49, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Jana, W.; Jacob, D.B.; Urs, B.; Derek, M.; Ting, R.; Alejiandra, T.; Foppe, S.; Branislav, V.; Fabrica, C.; Heidelore, F. PFAS Analysis in Water for the Global Monitoring Plan of the Stockholm Convention; United Nations Environment Programme (UNEP): Geneva, Switzerland, 2015. [Google Scholar]
- Polder, A.; Muller, M.B.; Lyche, J.L.; Mdegela, R.H.; Nonga, H.E.; Mabiki, F.P.; Mbise, T.J.; Skaare, J.U.; Sandvik, M.; Skjerve, E.; et al. Levels and patterns of persistent organic pollutants (POPs) in tilapia (Oreochromis sp.) from four different lakes in Tanzania: Geographical differences and implications for human health. Sci. Total Environ. 2014, 488–489, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [Green Version]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khezri, A.; Fraser, T.W.K.; Nourizadeh-Lillabadi, R.; Kamstra, J.H.; Berg, V.; Zimmer, K.E.; Ropstad, E. A Mixture of Persistent Organic Pollutants and Perfluorooctanesulfonic Acid Induces Similar Behavioural Responses, but Different Gene Expression Profiles in Zebrafish Larvae. Int. J. Mol. Sci. 2017, 18, 291. https://doi.org/10.3390/ijms18020291
Khezri A, Fraser TWK, Nourizadeh-Lillabadi R, Kamstra JH, Berg V, Zimmer KE, Ropstad E. A Mixture of Persistent Organic Pollutants and Perfluorooctanesulfonic Acid Induces Similar Behavioural Responses, but Different Gene Expression Profiles in Zebrafish Larvae. International Journal of Molecular Sciences. 2017; 18(2):291. https://doi.org/10.3390/ijms18020291
Chicago/Turabian StyleKhezri, Abdolrahman, Thomas W. K. Fraser, Rasoul Nourizadeh-Lillabadi, Jorke H. Kamstra, Vidar Berg, Karin E. Zimmer, and Erik Ropstad. 2017. "A Mixture of Persistent Organic Pollutants and Perfluorooctanesulfonic Acid Induces Similar Behavioural Responses, but Different Gene Expression Profiles in Zebrafish Larvae" International Journal of Molecular Sciences 18, no. 2: 291. https://doi.org/10.3390/ijms18020291






