Sphingosine Kinase-1 Involves the Inhibitory Action of HIF-1α by Chlorogenic Acid in Hypoxic DU145 Cells
Abstract
:1. Introduction
2. Results
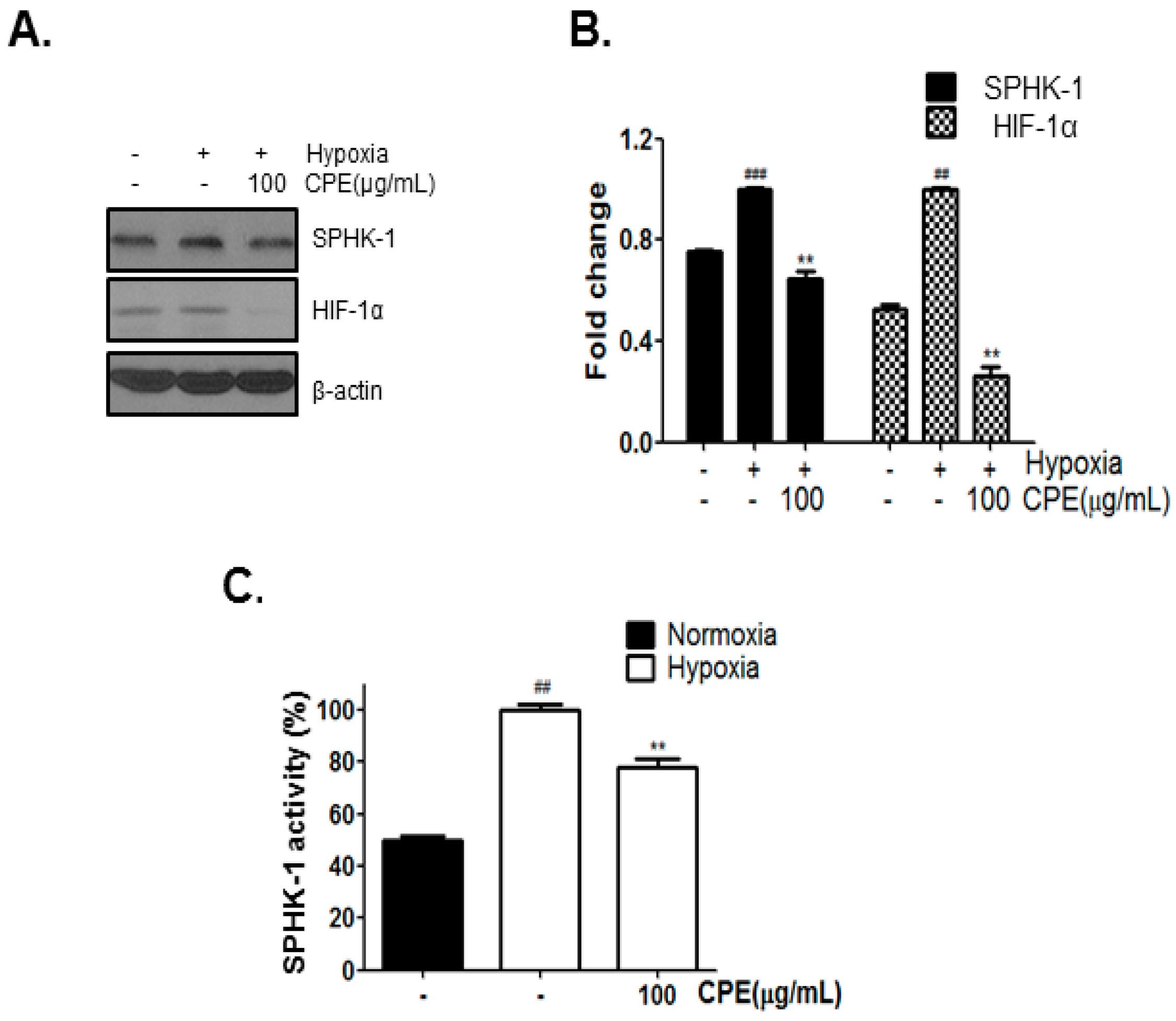
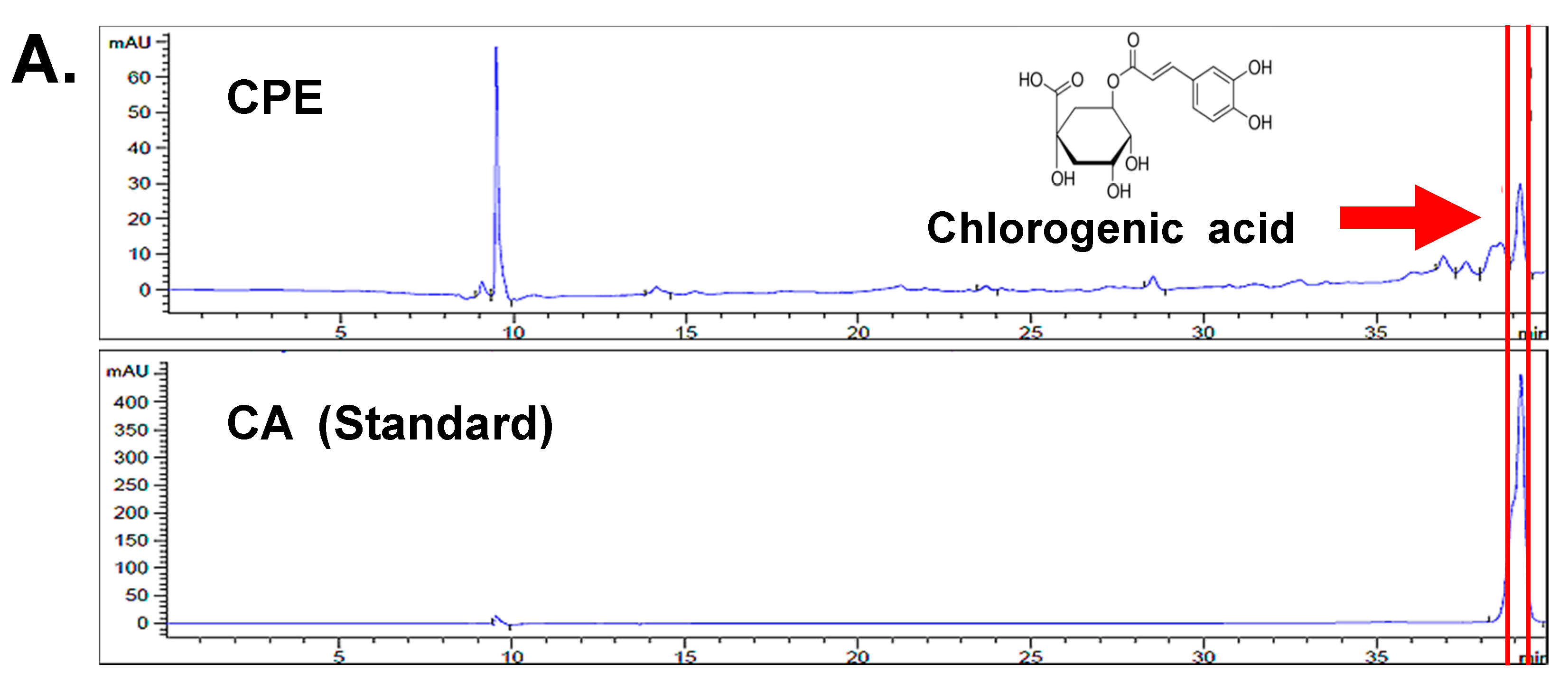
2.1. CPE Decreases HIF-1α and SPHK-1 Abundance in Hypoxic Condition
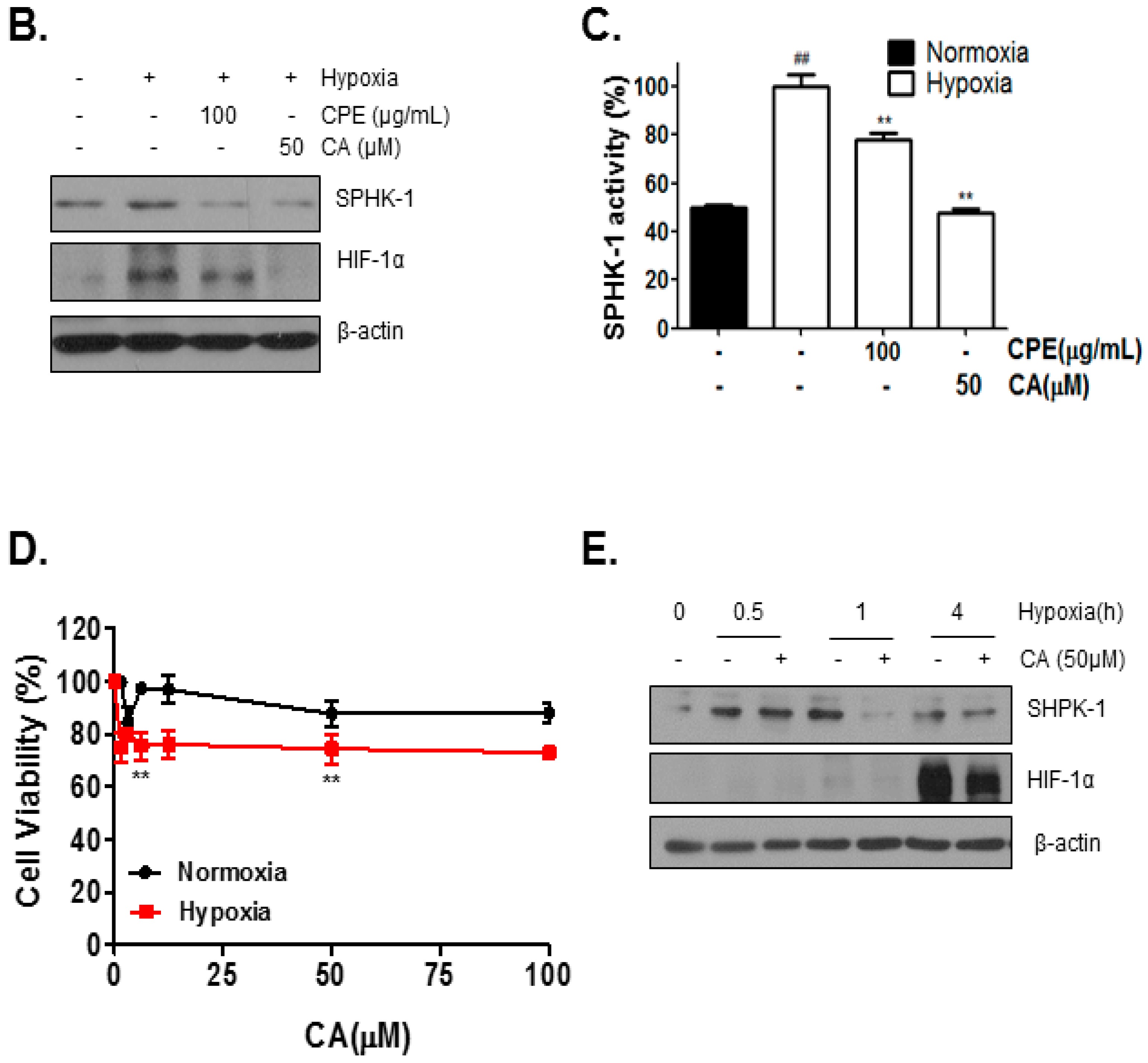
2.2. Chlorogenic Acid (CA), One of Four Major Compounds of CPE, Decreases HIF-1α and SPHK-1
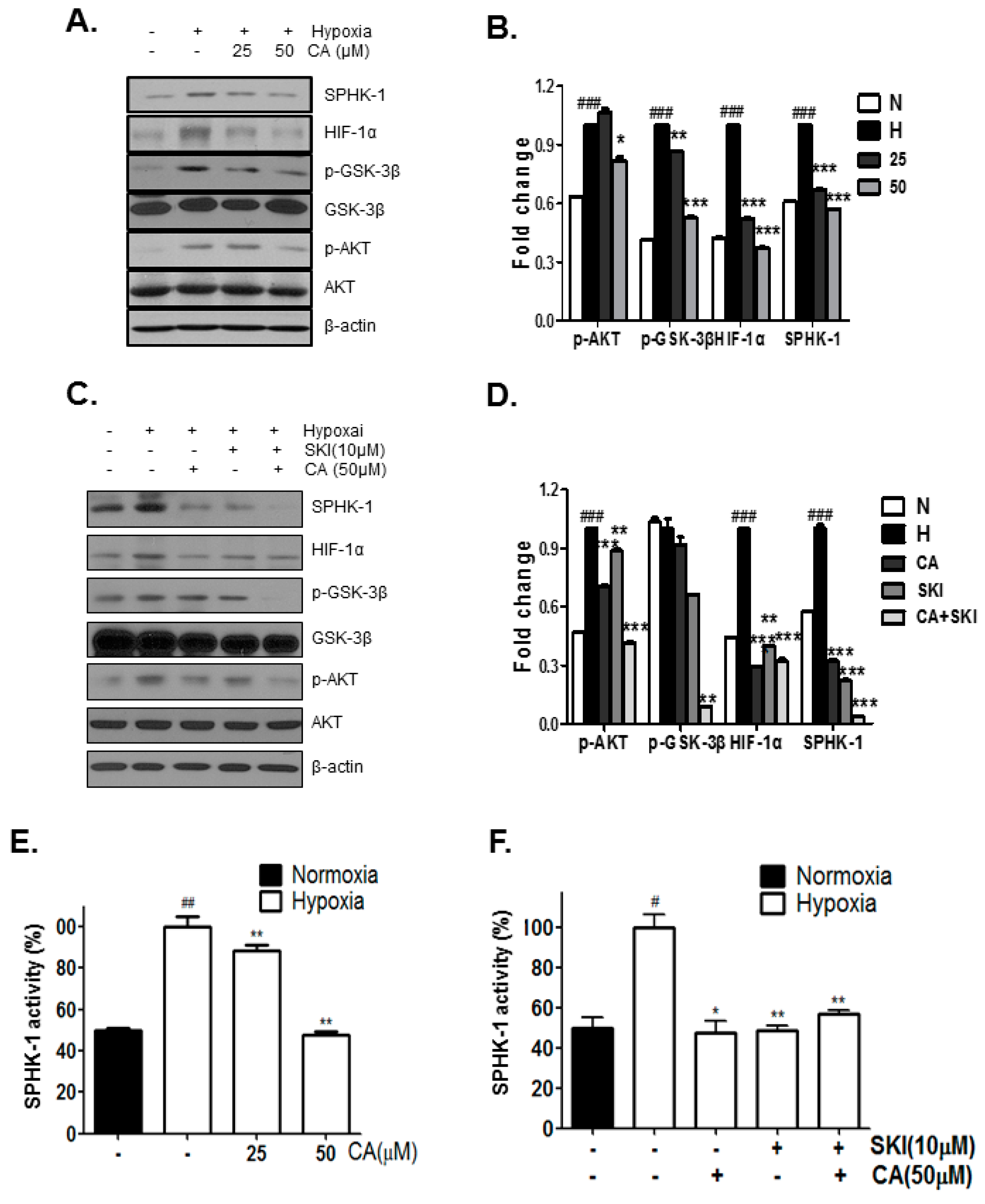
2.3. CA Inhibits Phosphorylation of AKT and GSK-3β, Which Are Involved in HIF-1α Stabilization, by SPHK-1
2.4. CA Inhibits Hypoxia-Induced Angiogenesis
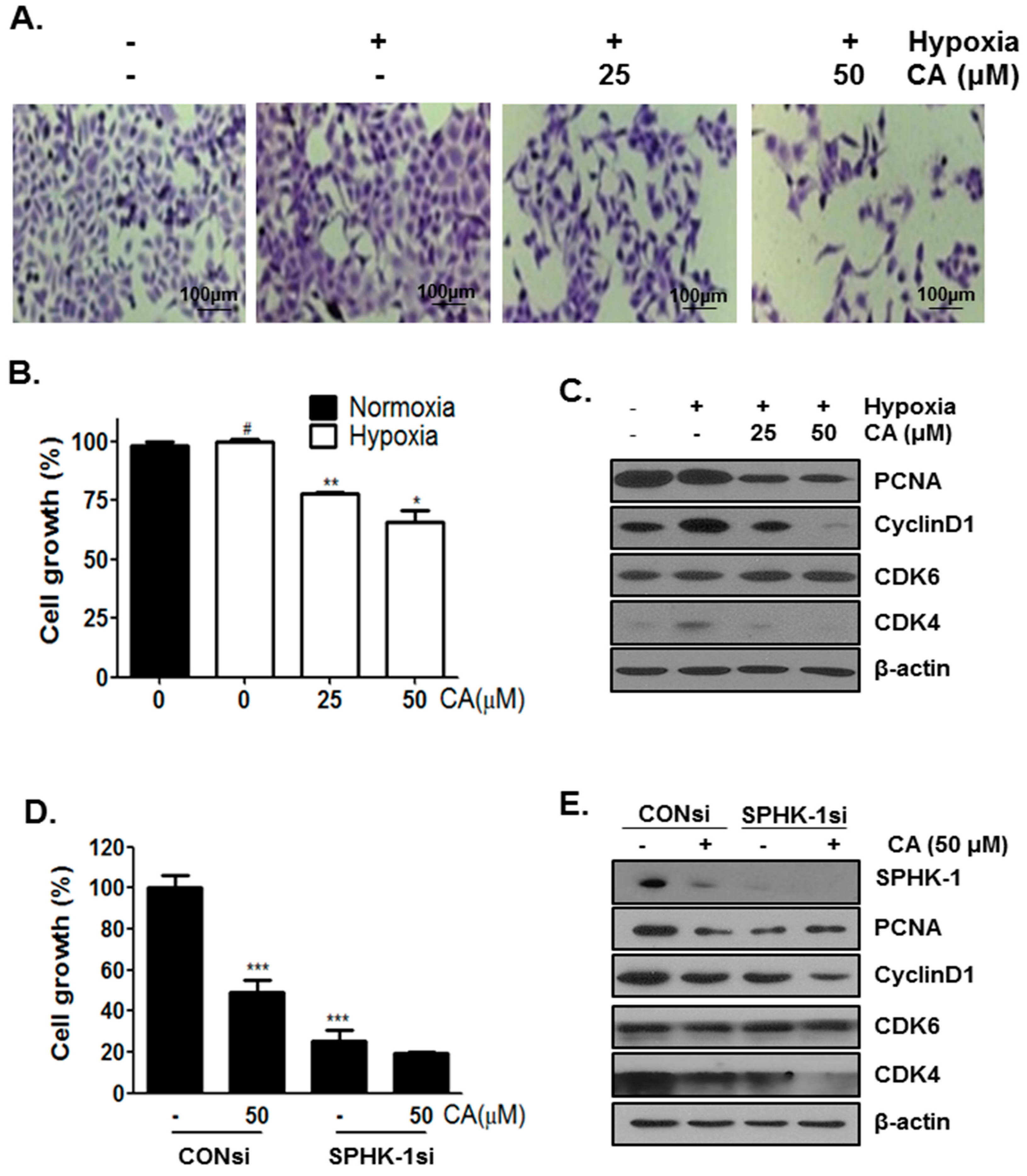
2.5. CA Inhibits Cell Proliferation in Hypoxic Condition
3. Discussion
4. Materials and Methods
4.1. Test Compound
4.2. Cell Culture Assay
4.3. Hypoxia Treatment
4.4. Cytotoxicity Assay
4.5. Western Blot Analysis
4.6. HPLC Analysis
4.7. Crystal Violet Staining
4.8. Sphingosine Kinase Activity Assay
4.9. Measurement of VEGF Production
4.10. SPHK-1 Gene Silencing
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CA | Chlorogenic acid |
| CPE | Crataegus Pinnatifida Bunge var. typical Schneider ethanol extract |
| SKI | SPHK inhibitor |
| SPHK-1 | Sphingosine kinase 1 |
| HIF-1α | Hypoxia-inducible factor-1α |
| PBS | Phosphate-buffered saline |
| OD | Optical density |
References
- Chang, Q.; Zuo, Z.; Harrison, F.; Chow, M.S. Hawthorn. J. Clin. Pharmacol. 2002, 42, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Kao, E.S.; Wang, C.J.; Lin, W.L.; Yin, Y.F.; Wang, C.P.; Tseng, T.H. Anti-inflammatory potential of flavonoid contents from dried fruit of crataegus pinnatifida in vitro and in vivo. J. Agric. Food Chem. 2005, 53, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Chen, C.; Chen, L.; Cheng, K.; Yeh, C.; Cheng, J. Decrease of blood lipids induced by Shan-Zha (fruit of Crataegus pinnatifida) is mainly related to an increase of PPARα in liver of mice fed high-fat diet. Horm. Metab. Res. 2011, 43, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlcek, J.; Balla, S.; Szekeres, L.; Adam, V.; Kizek, R. Polyphenolic profile and biological activity of chinese hawthorn (Crataegus pinnatifida Bunge) fruits. Molecules 2012, 17, 14490–14509. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, P.; Zhao, C.; Zhang, Y.; Liu, H.; Hu, L.; Gao, X.; Zhang, D. Prevention effect in selenite-induced cataract in vivo and antioxidative effects in vitro of crataegus pinnatifida leaves. Biol. Trace Elem. Res. 2011, 142, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Lee, M.Y.; Lim, H.S.; Ha, H.; Seo, C.S.; Kim, J.C.; Shin, H.K. An extract of crataegus pinnatifida fruit attenuates airway inflammation by modulation of matrix metalloproteinase-9 in ovalbumin induced asthma. PLoS ONE 2012, 7, e45734. [Google Scholar] [CrossRef] [PubMed]
- Kao, E.S.; Wang, C.J.; Lin, W.L.; Chu, C.Y.; Tseng, T.H. Effects of polyphenols derived from fruit of crataegus pinnatifida on cell transformation, dermal edema and skin tumor formation by phorbol ester application. Food Chem. Toxicol. 2007, 45, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.S.; Islam, M.N.; Jung, H.A.; Choi, J.S. In vitro antidiabetic potential of the fruits of crataegus pinnatifida. Res. Pharm. Sci. 2014, 9, 11–22. [Google Scholar] [PubMed]
- Zhang, J.; Liang, R.; Wang, L.; Yan, R.; Hou, R.; Gao, S.; Yang, B. Effects of an aqueous extract of Crataegus pinnatifida Bge. var. major N.E.Br. Fruit on experimental atherosclerosis in rats. J. Ethnopharm. 2013, 148, 563–569. [Google Scholar]
- Wen, L.; Guo, X.; Liu, R.H.; You, L.; Abbasi, A.M.; Fu, X. Phenolic contents and cellular antioxidant activity of chinese hawthorn “Crataegus pinnatifida”. Food Chem. 2015, 186, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Peng, W.; Qin, R.; Zhou, H. Crataegus pinnatifida: Chemical constituents, pharmacology, and potential applications. Molecules 2014, 19, 1685–1712. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Li, J.Z.; Kayahara, H.; Ma, L.; Wu, L.X.; Nakamura, K. Quantification of the polyphenols and triterpene acids in chinese hawthorn fruit by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 4574–4581. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Ient, J.; Gottgens, E.L.; Krieg, A.J.; Hammond, E.M. Epigenetic therapy for solid tumors: Highlighting the impact of tumor hypoxia. Genes 2015, 6, 935–956. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Jonathan, E.; Evans, S.M.; Koch, C.J.; Muschel, R.J.; McKenna, W.G.; Wu, J.; Bernhard, E.J. The farnesyltransferase inhibitor L744,832 reduces hypoxia in tumors expressing activated H-ras. Cancer Res. 2001, 61, 2289–2293. [Google Scholar] [PubMed]
- Chen, C.; Pore, N.; Behrooz, A.; Ismail-Beigi, F.; Maity, A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 2001, 276, 9519–9525. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Ader, I.; Bouquerel, P.; Brizuela, L.; Malavaud, B.; Mazerolles, C.; Rischmann, P. Activation of sphingosine kinase-1 in cancer: Implications for therapeutic targeting. Curr. Mol. Pharm. 2010, 3, 53–65. [Google Scholar] [CrossRef]
- Ader, I.; Malavaud, B.; Cuvillier, O. When the sphingosine kinase 1/sphingosine 1-phosphate pathway meets hypoxia signaling: New targets for cancer therapy. Cancer Res. 2009, 69, 3723–3726. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lee, H.J.; Jeong, S.J.; Kim, H.S.; Chen, C.Y.; Lee, E.O.; Kim, S.H. Sphingosine kinase 1 pathway is involved in melatonin-induced HIF-1α inactivation in hypoxic PC-3 prostate cancer cells. J. Pineal Res. 2011, 51, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Terashima, H.; Aizawa, S.; Taga, A.; Yamamoto, A.; Tsutsumiuchi, K.; Kodama, S. Simultaneous determination of trigonelline, caffeine, chlorogenic acid and their related compounds in instant coffee samples by HPLC using an acidic mobile phase containing octanesulfonate. Anal. Sci. 2015, 31, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Luo, T.; Wu, F.; Mei, Y.W.; Peng, J.; Liu, H.; Li, H.R.; Zhang, S.L.; Dong, J.H.; Fang, Y.; et al. Involvement of TLR2 and TLR9 in the anti-inflammatory effects of chlorogenic acid in HSV-1-infected microglia. Life Sci. 2015, 127, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. In vivo anti-diabetic potential of chlorogenic acid as a consequence of synergism with other phenolic compounds? Br. J. Nutr. 2015, 113, 546–547. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Zhang, J.F.; Chen, X.X.; Sun, P.L. Microwave-assisted extraction and purification of chlorogenic acid from by-products of eucommia ulmoides oliver and its potential anti-tumor activity. J. Food Sci. Technol. 2015, 52, 4925–4934. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Sheng, Y.C.; Jiang, P.; Wei, H.; Ji, L.L. Chlorogenic acid prevents acetaminophen-induced liver injury: The involvement of CYP450 metabolic enzymes and some antioxidant signals. J. Zhejiang Univ. Sci. B 2015, 16, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.Q.; Tang, Z.H.; Yan, Y.X.; Guo, C.R.; Cao, L.; Ding, G.; Huang, W.Z.; Wang, Z.Z.; Wang, K.D.; Xiao, W.; et al. Study on the anti-gout activity of chlorogenic acid: Improvement on hyperuricemia and gouty inflammation. Am. J. Chin. Med. 2014, 42, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Xiao, Y.; Lin, Y.; Mo, Z.; Chen, Y.; Peng, X.; Xiang, C.; Li, Y.; Li, W. Chlorogenic acid-enriched extract from Eucommia ulmoides leaves inhibits hepatic lipid accumulation through regulation of cholesterol metabolism in HepG2 cells. Pharm. Biol. 2016, 54, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Fu, Z.X.; Yao, J.; Ma, H.P. Effects of chlorogenic acid on the viability and HIF-1α mRNA expression of PC12 cells exposed to hypoxia. Zhong Yao Cai 2013, 36, 1644–1647. [Google Scholar] [PubMed]
- Park, J.J.; Hwang, S.J.; Park, J.H.; Lee, H.J. Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway. Cell. Oncol. 2015, 38, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Du, W.Y.; Chang, C.; Zhang, Y.; Liu, Y.Y.; Sun, K.; Wang, C.S.; Wang, M.X.; Liu, Y.; Wang, F.; Fan, J.Y.; et al. High-dose chlorogenic acid induces inflammation reactions and oxidative stress injury in rats without implication of mast cell degranulation. J. Ethnopharm. 2013, 147, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, M.; Nakaso, K.; Kusumoto, C.; Katano, S.; Tajima, N.; Yamashita, A.; Zushi, T.; Ito, S.; Matsura, T. Cytoprotective effect of chlorogenic acid against α-synuclein-related toxicity in catecholaminergic PC12 cells. J. Clin. Biochem. Nutr. 2012, 51, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Majhi, S.; Saha, B.P.; Mukherjee, P.K. Chlorogenic acid-phospholipid complex improve protection against UVA induced oxidative stress. J. Photochem. Photobiol. B 2014, 130, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Mottet, D.; Dumont, V.; Deccache, Y.; Demazy, C.; Ninane, N.; Raes, M.; Michiels, C. Regulation of hypoxia-inducible factor-1α protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/AKT/glycogen synthase kinase 3β pathway in HepG2 cells. J. Biol. Chem. 2003, 278, 31277–31285. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Chen, W.W.; Zhang, X. Glioblastoma multiforme: Effect of hypoxia and hypoxia inducible factors on therapeutic approaches. Oncol. Lett. 2016, 12, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Nan, K.J. Activation of PI3 kinase/AKT/HIF-1α pathway contributes to hypoxia-induced epithelial-mesenchymal transition and chemoresistance in hepatocellular carcinoma. Int. J. Oncol. 2012, 40, 461–468. [Google Scholar] [PubMed]
- Sun, Y.; Guan, Z.; Liang, L.; Cheng, Y.; Zhou, J.; Li, J.; Xu, Y. HIF-1α/MDR1 pathway confers chemoresistance to cisplatin in bladder cancer. Oncol. Rep. 2016, 35, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Alshaker, H.; Wang, Q.; Kawano, Y.; Arafat, T.; Bohler, T.; Winkler, M.; Cooper, C.; Pchejetski, D. Everolimus (rad001) sensitizes prostate cancer cells to docetaxel by down-regulation of HIF-1α and sphingosine kinase 1. Oncotarget 2016, 7, 80943–80956. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Cho, S.; Park, E.; Kim, B.; Sohn, E.J.; Oh, B.; Lee, E.O.; Lee, H.J.; Kim, S.H. Coumestrol suppresses hypoxia inducible factor 1α by inhibiting ROS mediated sphingosine kinase 1 in hypoxic PC-3 prostate cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 2560–2564. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Kim, J.S.; Lee, M.S.; Lee, H.J. Anti-cancer effect of pristimerin by inhibition of HIF-1α involves the SPHK-1 pathway in hypoxic prostate cancer cells. BMC Cancer 2016, 16, 701. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-S.; Lee, S.-O.; Kim, K.-R.; Lee, H.-J. Sphingosine Kinase-1 Involves the Inhibitory Action of HIF-1α by Chlorogenic Acid in Hypoxic DU145 Cells. Int. J. Mol. Sci. 2017, 18, 325. https://doi.org/10.3390/ijms18020325
Lee M-S, Lee S-O, Kim K-R, Lee H-J. Sphingosine Kinase-1 Involves the Inhibitory Action of HIF-1α by Chlorogenic Acid in Hypoxic DU145 Cells. International Journal of Molecular Sciences. 2017; 18(2):325. https://doi.org/10.3390/ijms18020325
Chicago/Turabian StyleLee, Myoung-Sun, Seon-Ok Lee, Kyu-Ri Kim, and Hyo-Jeong Lee. 2017. "Sphingosine Kinase-1 Involves the Inhibitory Action of HIF-1α by Chlorogenic Acid in Hypoxic DU145 Cells" International Journal of Molecular Sciences 18, no. 2: 325. https://doi.org/10.3390/ijms18020325





