Antidepressant Effects of Aripiprazole Augmentation for Cilostazol-Treated Mice Exposed to Chronic Mild Stress after Ischemic Stroke
Abstract
:1. Introduction
2. Results
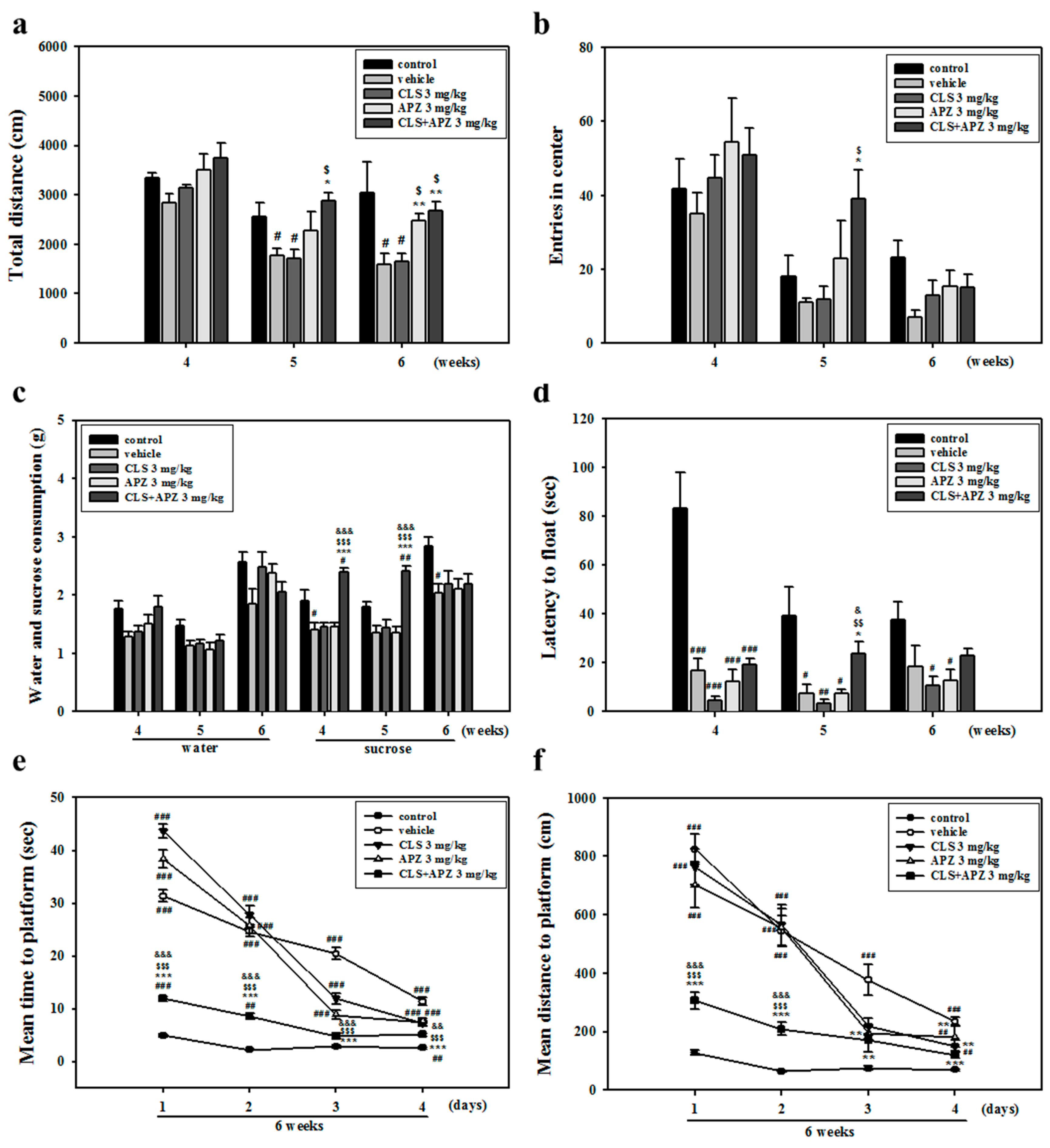
2.1. Treatment Effects on the Depressive Behavioral Phenotypes
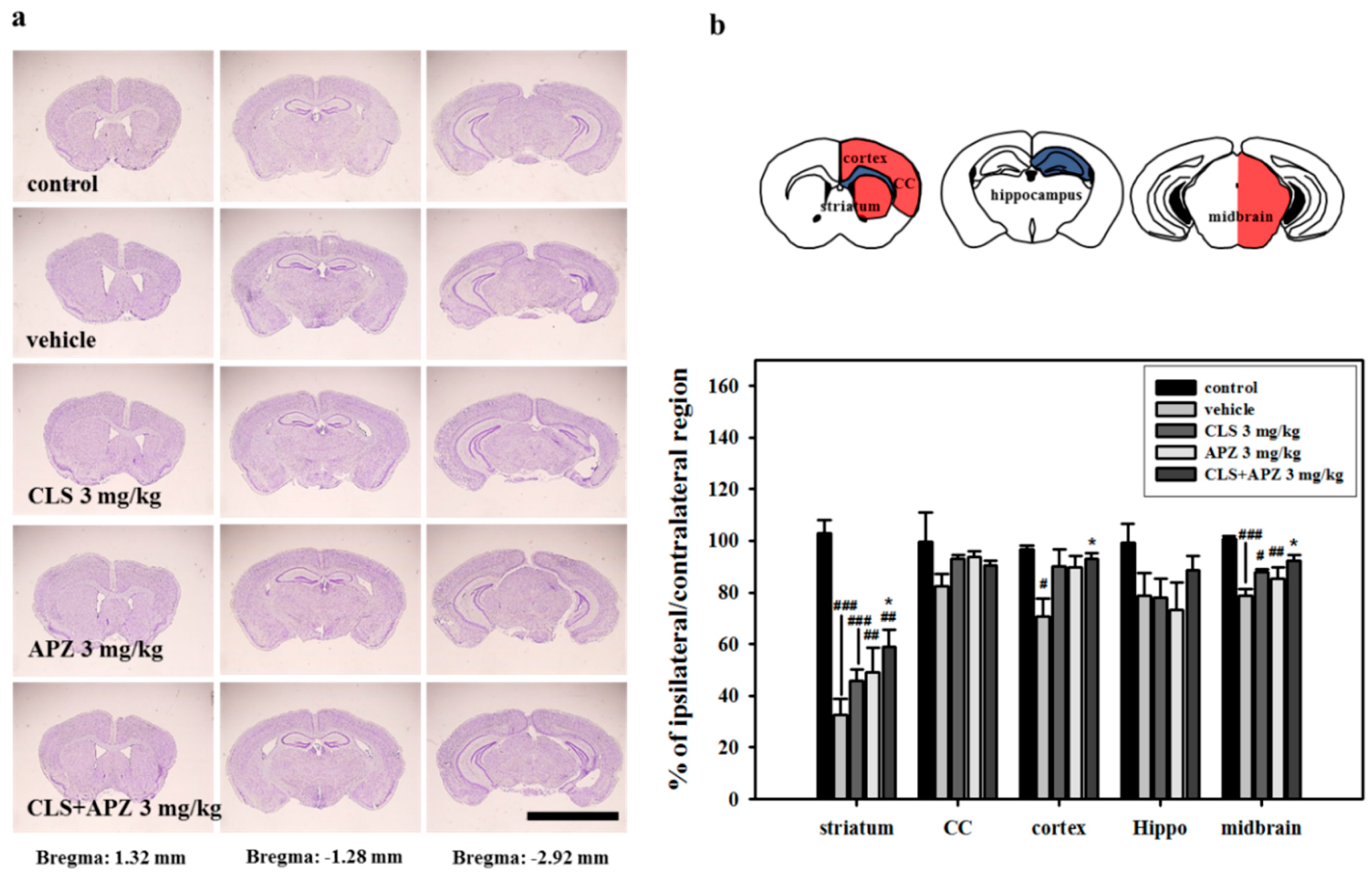
2.2. Treatment Effects on the Atrophic Changes of the Brain
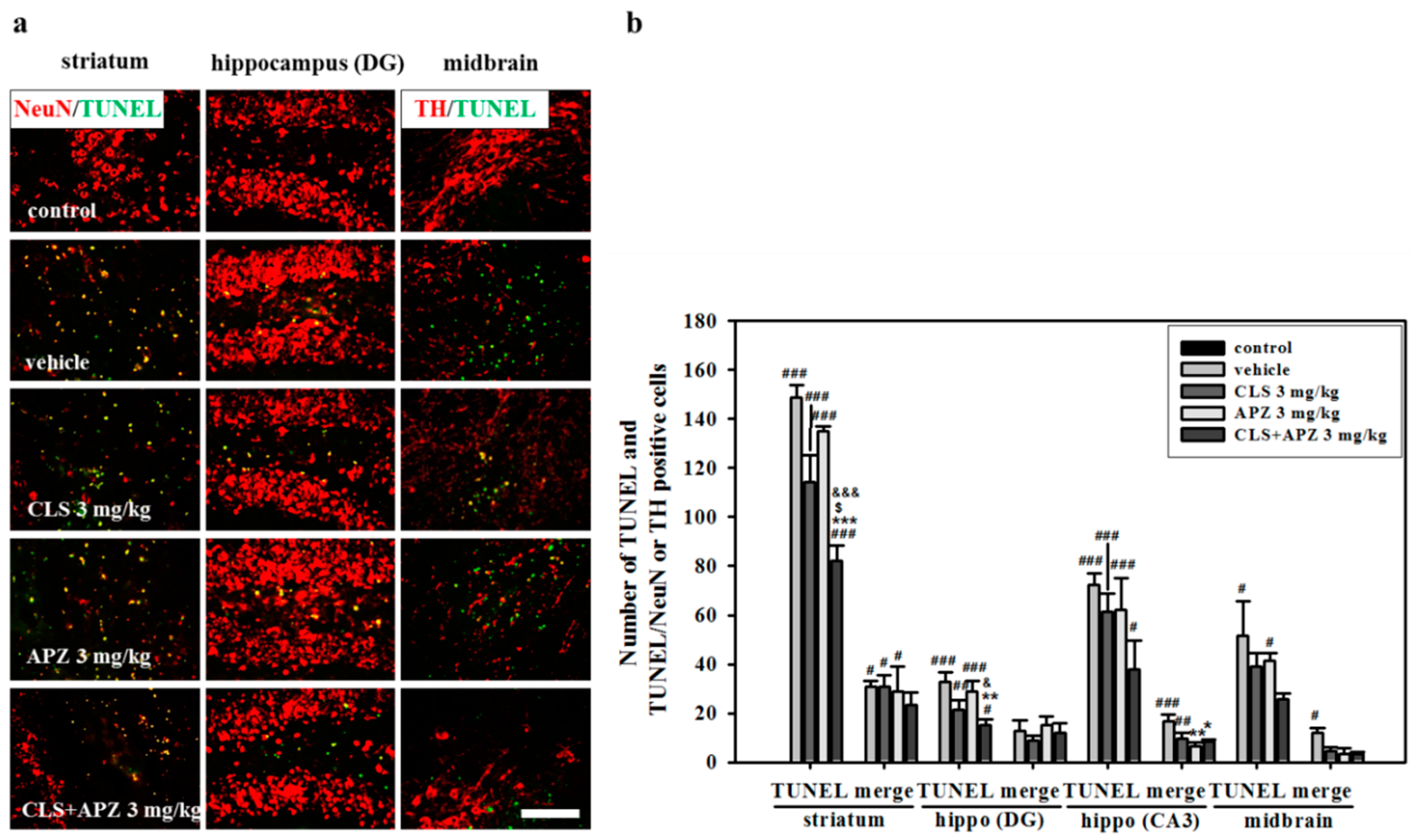
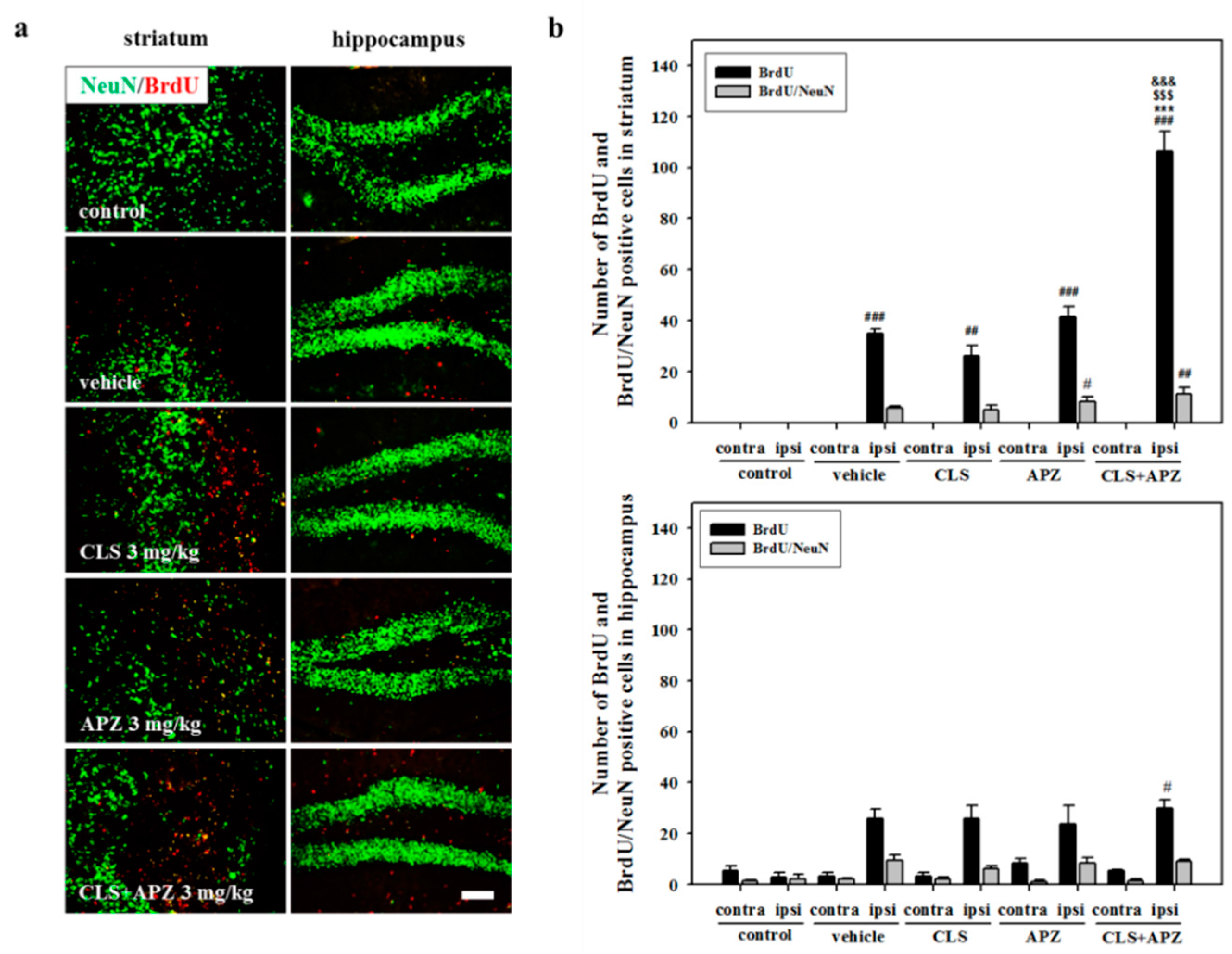
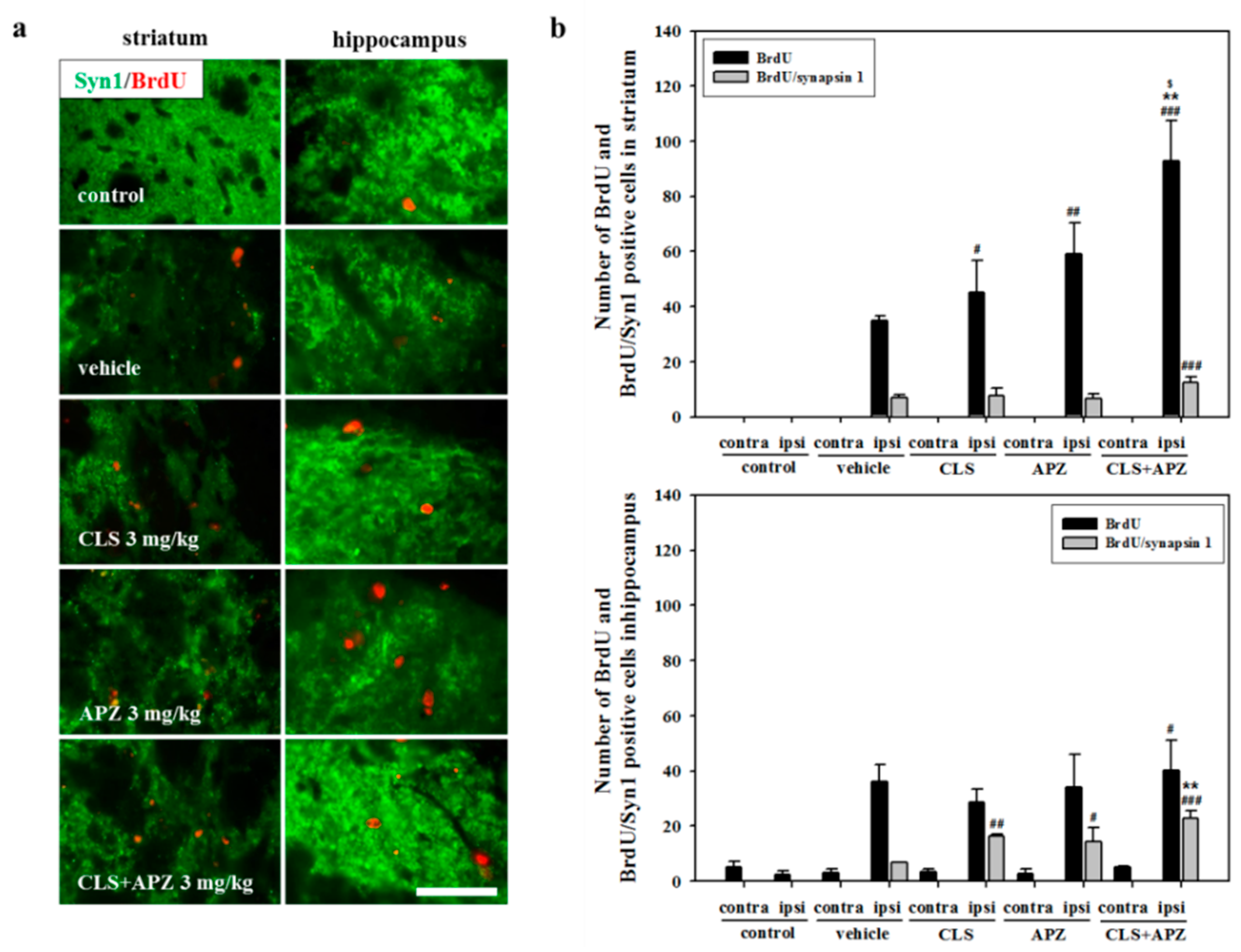
2.3. Treatment Effects on Proliferation, Differentiation, and Synaptic Formation in Neuronal Progenitor Cells
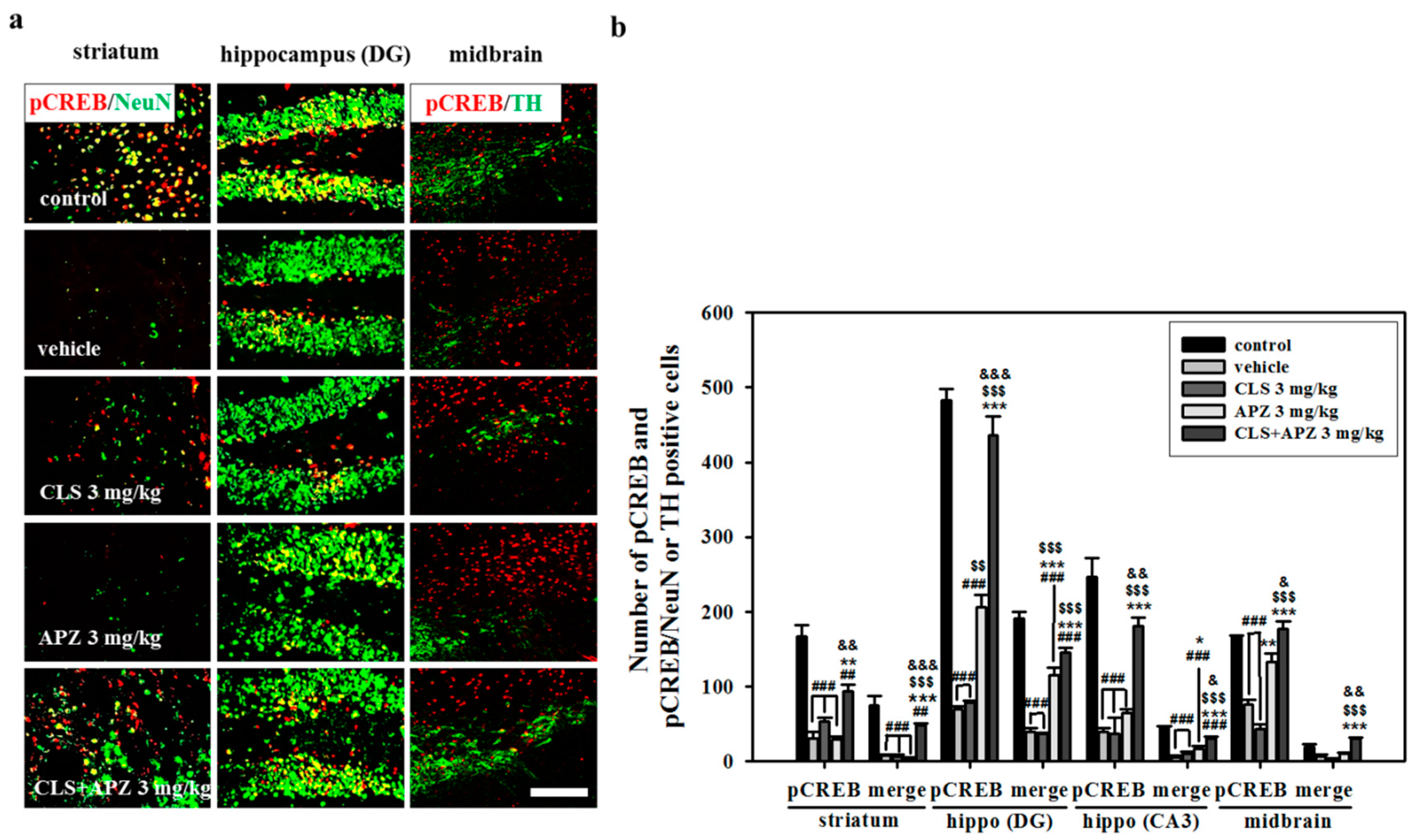
2.4. Effect on Phosphorylation of CREB
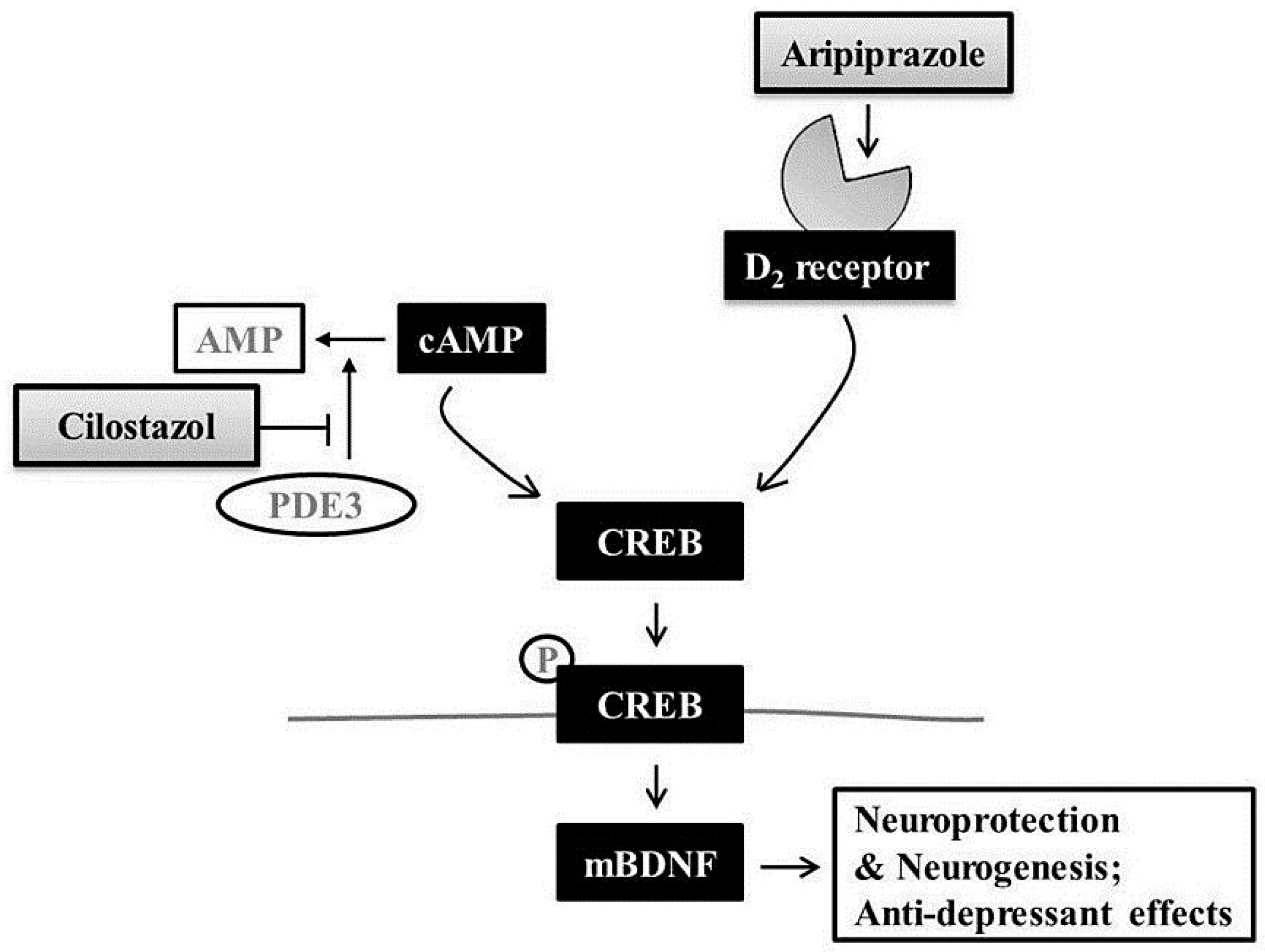
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Focal Cerebral Ischemia
4.3. Chronic Mild Stress
4.4. Drug Administration
4.5. Bromodeoxyuridine Labeling
4.6. Behavioral Experiments
4.6.1. Open Field Test
4.6.2. Sucrose Preference Test
4.6.3. Forced Swim Test
4.6.4. Morris Water Maze Test
4.7. Histological Assessment
4.8. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling Assay
4.9. Immunofluorescence
4.10. Data Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| APZ | aripiprazole |
| BDNF | brain-derived neurotrophic factor |
| BrdU | 5-bromo-2′-deoxyuridine |
| CA | cornu ammonis |
| CaMKII | calcium/calmodulin-dependent protein kinase II |
| cAMP | cyclic adenosine monophosphate |
| CLS | cilostazol |
| CMS | chronic mild stress |
| CREB | cAMP response element binding protein |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DG | dentate gyrus |
| MCA | middle cerebral artery |
| MCAO | middle cerebral artery occlusion |
| NeuN | neuronal nuclei |
| PBS | phosphate buffered saline |
| PDE3 | type 3 phosphodiesterase |
| PP1 | protein phosphatase 1 |
| Syn1 | synapsin 1 |
| TH | tyrosine hydroxylase |
| TUNEL | terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling |
References
- Kronenberg, G.; Gertz, K.; Heinz, A.; Endres, M. Of mice and men: Modelling post-stroke depression experimentally. Br. J. Pharmacol. 2014, 171, 4673–4689. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Meyers, B.S.; Young, R.C.; Campbell, S.; Silbersweig, D.; Charlson, M. “Vascular depression” hypothesis. Arch. Gen. Psychiatry 1997, 54, 915–922. [Google Scholar] [CrossRef]
- Gothe, F.; Enache, D.; Wahlund, L.O.; Winblad, B.; Crisby, M.; Lokk, J.; Aarsland, D. Cerebrovascular diseases and depression: Epidemiology, mechanisms and treatment. Panminerva Med. 2012, 54, 161–170. [Google Scholar] [PubMed]
- Husseini, L.; Saleh, A.; Reifenberger, G.; Hartung, H.P.; Kieseier, B.C. Inflammatory demyelinating brain lesions heralding primary CNS lymphoma. Can. J. Neurol. Sci. 2012, 39, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Buga, A.M.; Tica, A.A.; Albu, C.V. Perfusion deficits, inflammation and aging precipitate depressive behaviour. Biogerontology 2014, 15, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.K.; Alkon, D.L. Cerebral ischemia-induced difference in sensitivity to depression and potential therapeutics in rats. Behav. Pharmacol. 2013, 24, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, I.; Kronenberg, G.; Endres, M.; Schumann-Bard, P.; Freret, T.; Filipkowski, R.K.; Kaczmarek, L.; Popa-Wagner, A. Post-stroke depression: Mechanisms, translation and therapy. J. Cell. Mol. Med. 2012, 16, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Sarkisyan, G.; Roberts, A.J.; Hedlund, P.B. The 5-HT7 receptor as a mediator and modulator of antidepressant-like behavior. Behav. Brain Res. 2010, 209, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sarkisova, K.; van Luijtelaar, G. The WAG/Rij strain: A genetic animal model of absence epilepsy with comorbidity of depression [corrected]. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S. Frontostriatal and limbic dysfunction in late-life depression. Am. J. Geriatr. Psychiatry 2002, 10, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, L.L.; le Masurier, M.; Ebmeier, K.P. White matter hyperintensities in late life depression: A systematic review. J. Neurol. Neurosurg. Psychiatry 2008, 79, 619–624. [Google Scholar] [CrossRef]
- Burda, K.; Czubak, A.; Kus, K.; Nowakowska, E.; Ratajczak, P.; Zin, J. Influence of aripiprazole on the antidepressant, anxiolytic and cognitive functions of rats. Pharmacol. Rep. 2011, 63, 898–907. [Google Scholar] [CrossRef]
- Russo, E.; Citraro, R.; Davoli, A.; Gallelli, L.; Di Paola, E.D.; De Sarro, G. Ameliorating effects of aripiprazole on cognitive functions and depressive-like behavior in a genetic rat model of absence epilepsy and mild-depression comorbidity. Neuropharmacology 2013, 64, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Kimura, M. Two cases of emotional disorder after middle cerebral artery infarction showing distinct responses to antidepressant treatment. Neuropsychiatr. Dis. Treat. 2014, 10, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, J.; Liu, Y.; Sun, B.; Shakur, Y.; Yoshitake, M.; Czerwiec, F. Cilostazol as a unique antithrombotic agent. Curr. Pharm. Des. 2003, 9, 2289–2302. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tanaka, R.; Liu, M.; Hattori, N.; Urabe, T. Cilostazol attenuates ischemic brain injury and enhances neurogenesis in the subventricular zone of adult mice after transient focal cerebral ischemia. Neuroscience 2010, 171, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.J.; Lee, E.J.; Kim, M.K.; Kim, S.Y.; Kim, J.N.; Kim, J.O.; Kim, H.J.; Kim, H.Y.; Han, J.S.; Shin, C.Y.; et al. Diabetes augments cognitive dysfunction in chronic cerebral hypoperfusion by increasing neuronal cell death: Implication of cilostazol for diabetes mellitus-induced dementia. Neurobiol. Dis. 2015, 73, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Yagita, Y.; Kawamura, M.; Sugiyama, Y.; Terasaki, Y.; Omura-Matsuoka, E.; Sasaki, T.; Kitagawa, K. Cilostazol, not aspirin, reduces ischemic brain injury via endothelial protection in spontaneously hypertensive rats. Stroke 2011, 42, 2571–2577. [Google Scholar] [CrossRef] [PubMed]
- Venna, V.R.; Weston, G.; Benashski, S.E.; Tarabishy, S.; Liu, F.; Li, J.; Conti, L.H.; McCullough, L.D. NF-κB contributes to the detrimental effects of social isolation after experimental stroke. Acta Neuropathol. 2012, 124, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Bellisario, V.; Capoccia, S.; Tirassa, P.; Calza, A.; Alleva, E.; Cirulli, F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology 2012, 37, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Kim, H.N.; Pak, M.E.; Ahn, S.M.; Hong, K.H.; Shin, H.K.; Choi, B.T. Studies on the animal model of post-stroke depression and application of antipsychotic aripiprazole. Behav. Brain Res. 2015, 287, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, G.; Balkaya, M.; Prinz, V.; Gertz, K.; Ji, S.; Kirste, I.; Heuser, I.; Kampmann, B.; Hellmann-Regen, J.; Gass, P.; et al. Exofocal dopaminergic degeneration as antidepressant target in mouse model of poststroke depression. Biol. Psychiatry 2012, 72, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Shin, H.K.; Kim, K.Y.; Lee, J.H.; Hong, K.W. Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J. Pharmacol. Exp. Ther. 2002, 300, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Katayama, Y.; Uchiyama, S.; Yamaguchi, T.; Handa, S.; Matsuoka, K.; Ohashi, Y.; Tanahashi, N.; Yamamoto, H.; Genka, C.; et al. Cilostazol for prevention of secondary stroke (CSPS 2): An aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010, 9, 959–968. [Google Scholar] [CrossRef]
- Shioda, N.; Sawai, M.; Ishizuka, Y.; Shirao, T.; Fukunaga, K. Nuclear translocation of calcium/calmodulin-dependent protein kinase IIδ3 promoted by protein phosphatase-1 enhances brain-derived neurotrophic factor expression in dopaminergic neurons. J. Biol. Chem. 2015, 290, 21663–21675. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, M.; Elbe, D. Focus on aripiprazole: A review of its use in child and adolescent psychiatry. J. Can. Acad. Child Adolesc. Psychiatry 2009, 18, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Umene-Nakano, W.; Yoshimura, R.; Okamoto, T.; Hori, H.; Nakamura, J. Aripiprazole improves various cognitive and behavioral impairments after traumatic brain injury: A case report. Gen. Hosp. Psychiatry 2013, 35, 103.e7–103.e9. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.; Reiter, R.J. Melatonin maintains adult hippocampal neurogenesis and cognitive functions after irradiation. Prog. Neurobiol. 2010, 90, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Hasebe, S.; Kawamoto, N.; Shiba, T.; Yamaguchi, T.; Kikuta, M.; Shuto, M.; Ogita, K. Beneficial in vivo effect of aripiprazole on neuronal regeneration following neuronal loss in the dentate gyrus: Evaluation using a mouse model of trimethyltin-induced neuronal loss/self-repair in the dentate gyrus. J. Pharmacol. Sci. 2014, 124, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, G.R.; Popa-Wagner, A.; Stanciulescu, E.C.; Babadan, L.; Buga, A.M. Post-stroke depression and the aging brain. J. Mol. Psychiatry 2013, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Koprivica, V.; Regardie, K.; Wolff, C.; Fernalld, R.; Murphy, J.J.; Kambayashi, J.; Kikuchi, T.; Jordan, S. Aripiprazole protects cortical neurons from glutamate toxicity. Eur. J. Pharmacol. 2011, 651, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Yan, B.C.; Park, J.H.; Ahn, J.H.; Lee, D.H.; Kim, I.H.; Cho, J.H.; Lee, J.C.; Kim, S.K.; Lee, B.; et al. Aripiprazole, an atypical antipsychotic drug, improves maturation and complexity of neuroblast dendrites in the mouse dentate gyrus via increasing superoxide dismutases. Neurochem. Res. 2013, 38, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, K.Y.; Lee, Y.K.; Park, S.Y.; Kim, C.D.; Lee, W.S.; Rhim, B.Y.; Hong, K.W. Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J. Pharmacol. Exp. Ther. 2004, 308, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Ishigooka, J.; Imamura, Y.; Ihara, S. Cilostazol, a cAMP phosphodiesterase 3 inhibitor, in the treatment of poststroke depression. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, S.; Malaterre, J.; Hollande, F.; Darcy, P.K.; Ramsay, R.G.; Mantamadiotis, T. cAMP response element binding protein is required for mouse neural progenitor cell survival and expansion. Stem Cells 2009, 27, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, S.Y.; Shin, Y.W.; Hong, K.W.; Kim, C.D.; Sung, S.M.; Kim, K.Y.; Lee, W.S. Neuroprotection by cilostazol, a phosphodiesterase type 3 inhibitor, against apoptotic white matter changes in rat after chronic cerebral hypoperfusion. Brain Res. 2006, 1082, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Tanaka, R.; Zhang, N.; Shimura, H.; Onodera, M.; Mochizuki, H.; Hattori, N.; Urabe, T. Crucial role for ser133-phosphorylated form of cyclic AMP-responsive element binding protein signaling in the differentiation and survival of neural progenitors under chronic cerebral hypoperfusion. Neuroscience 2009, 162, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Lee, C.H.; Lee, J.G.; Kim, L.W.; Shin, B.S.; Lee, B.J.; Kim, Y.H. Protective effects of atypical antipsychotic drugs against MPP+-induced oxidative stress in PC12 cells. Neurosci. Res. 2011, 69, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Eren, I.; Naziroglu, M.; Demirdas, A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem Res. 2007, 32, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.P.; Guthrie, C.R.; Wailes, L.M.; Zhao, X.; Means, A.R.; McKnight, G.S. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol. Cell. Biol. 1994, 14, 6107–6116. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, A.; Ogawa, Y.; Kitani, T.; Fujisawa, H.; Hagiwara, M. Calmodulin-dependent protein kinase II potentiates transcriptional activation through activating transcription factor 1 but not camp response element-binding protein. J. Biol. Chem. 1996, 271, 17957–17960. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Pham, L.D.; Hayakawa, K.; Matsuzaki, T.; Seo, J.H.; Magnain, C.; Ayata, C.; Kim, K.W.; Boas, D.; Lo, E.H.; et al. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 2013, 44, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Kim, H.N.; Hong, K.W.; Shin, H.K.; Choi, B.T. Anti-depressant effects of phosphodiesterase 3 inhibitor cilostazol in chronic mild stress-treated mice after ischemic stroke. Psychopharmacology 2015, 233, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Kamei, J.; Miyata, S.; Sunohara, T.; Kamei, A.; Shimada, M.; Ohsawa, M. Potentiation of the antidepressant-like effect of fluoxetine by aripiprazole in the mouse tail suspension test. J. Pharmacol. Sci. 2008, 108, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Towell, A.; Sampson, D.; Sophokleous, S.; Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 1987, 93, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Russo, S.J.; Ferguson, D.; Nestler, E.J.; Duman, R.S. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. USA 2010, 107, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jang, D.J.; Lee, N.; Ko, H.G.; Kim, H.; Kim, Y.S.; Kim, B.; Son, J.; Kim, S.H.; Chung, H.; et al. Induction of neuronal vascular endothelial growth factor expression by cAMP in the dentate gyrus of the hippocampus is required for antidepressant-like behaviors. J. Neurosci. 2009, 29, 8493–8505. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.R.; Kim, H.N.; Hong, K.W.; Shin, H.K.; Choi, B.T. Antidepressant Effects of Aripiprazole Augmentation for Cilostazol-Treated Mice Exposed to Chronic Mild Stress after Ischemic Stroke. Int. J. Mol. Sci. 2017, 18, 355. https://doi.org/10.3390/ijms18020355
Kim YR, Kim HN, Hong KW, Shin HK, Choi BT. Antidepressant Effects of Aripiprazole Augmentation for Cilostazol-Treated Mice Exposed to Chronic Mild Stress after Ischemic Stroke. International Journal of Molecular Sciences. 2017; 18(2):355. https://doi.org/10.3390/ijms18020355
Chicago/Turabian StyleKim, Yu Ri, Ha Neui Kim, Ki Whan Hong, Hwa Kyoung Shin, and Byung Tae Choi. 2017. "Antidepressant Effects of Aripiprazole Augmentation for Cilostazol-Treated Mice Exposed to Chronic Mild Stress after Ischemic Stroke" International Journal of Molecular Sciences 18, no. 2: 355. https://doi.org/10.3390/ijms18020355





