Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signaling Pathway
Abstract
:1. Introduction
2. Results
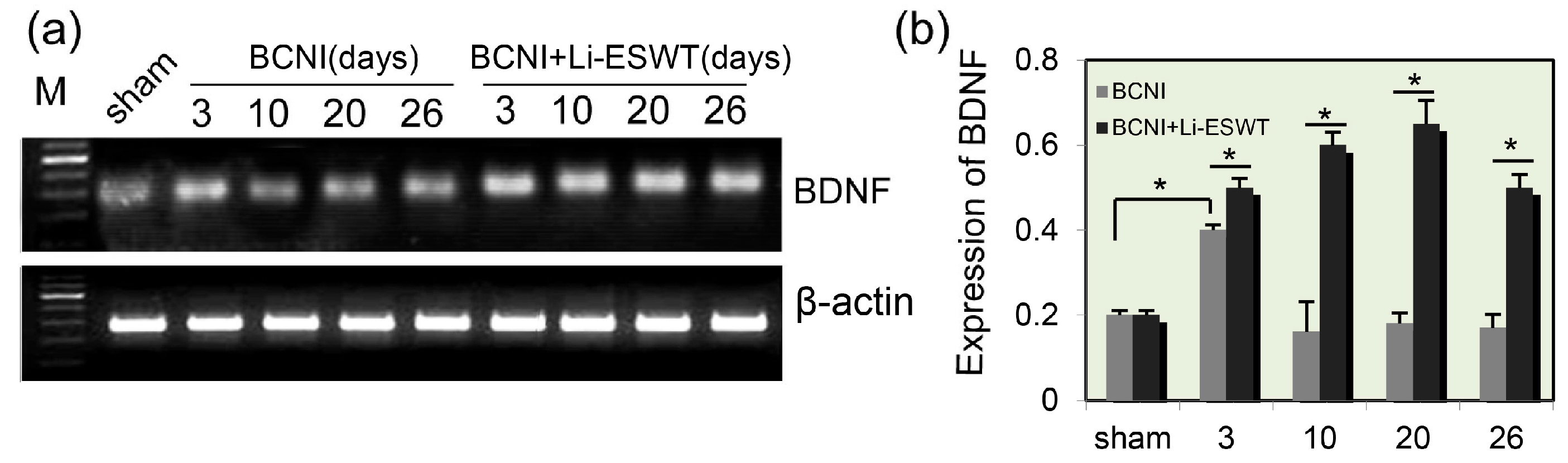
2.1. Expression Levels of BDNF in Penis Were Significantly Increased by Li-ESWT after Nerve Injury
2.2. Li-ESWT Increased Expression of BDNF in RT4-D6P2T Schwann Cells by Activating PERK/ATF4
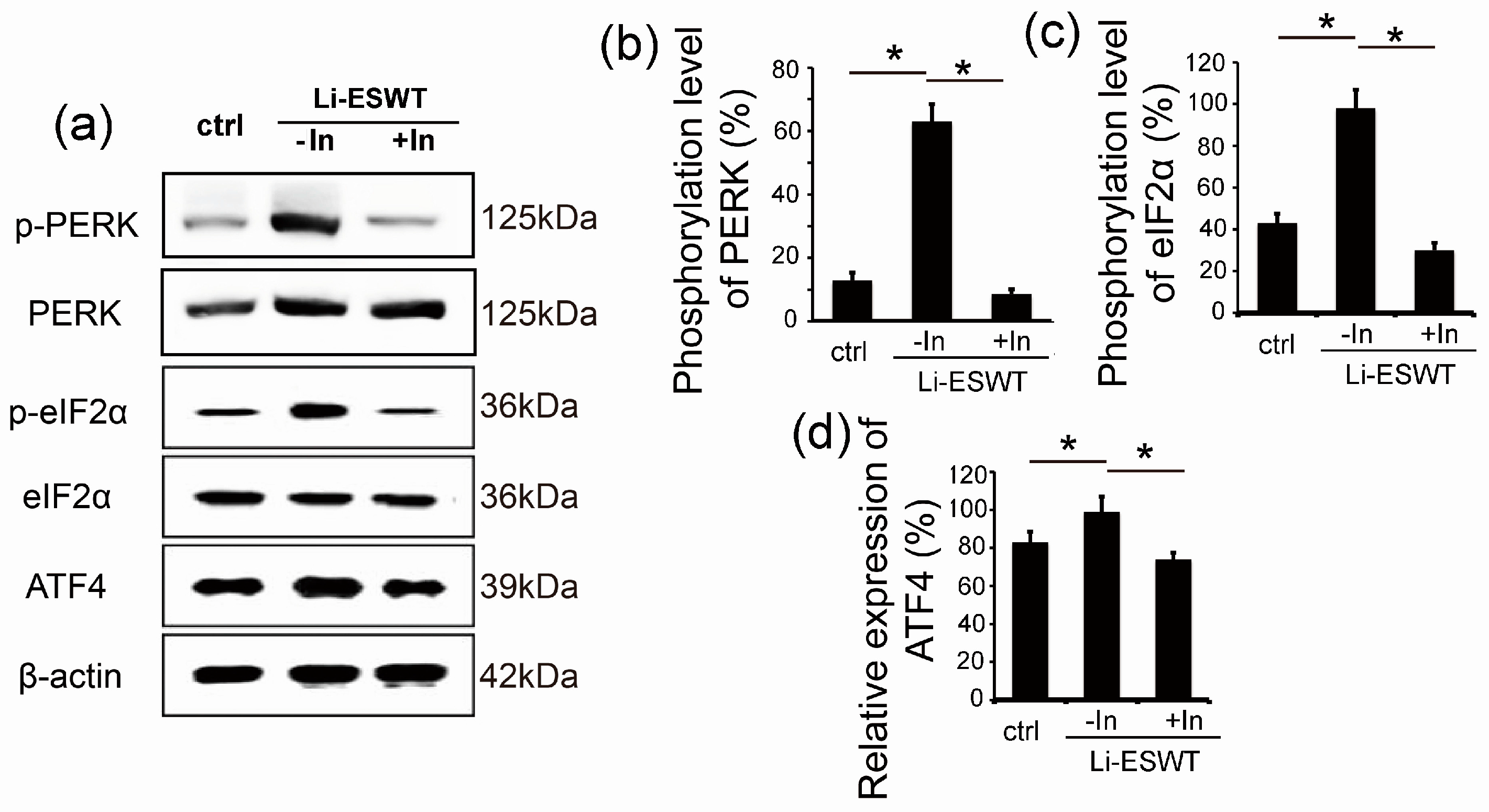
2.3. GSK2656157 Effectively Inhibited the Effect of Li-ESWT on the Phosphorylation of PERK and eIF2α and the Expression of ATF4 in RT4-D6P2T
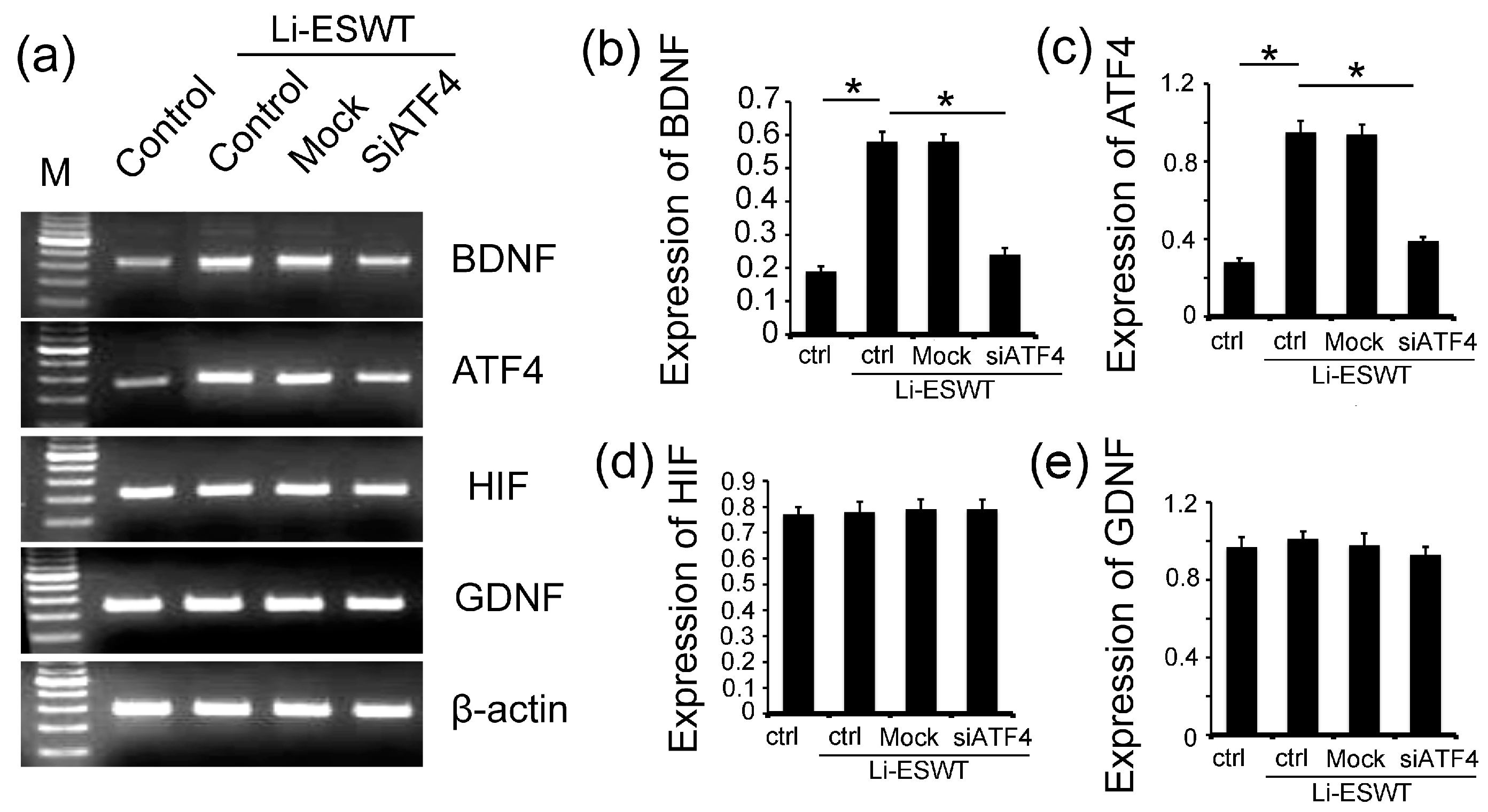
2.4. The Silencing of ATF4 Attenuated the Effect of Li-ESWT on the Expression of BDNF Dramatically, but Had No Effect on HIF1α and GDNF in RT4-D6P2T
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. BCNI Injury Model Establishment
4.3. Low-Intensity Extracorporeal Shock Wave Treatment
4.4. Rat Schwann Cell Treatment
4.5. Reverse Transcription-Polymerase Chain Reaction
4.6. Western Blotting
4.7. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bella, A.J.; Lin, G.; Garcia, M.M.; Tantiwongse, K.; Brant, W.O.; Lin, C.S.; Lue, T.F. Upregulation of penile brain-derived neurotrophic factor (BDNF) and activation of the JAK/STAT signalling pathway in the major pelvic ganglion of the rat after cavernous nerve transection. Eur. Urol. 2007, 52, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Bella, A.J.; Lin, G.; Tantiwongse, K.; Garcia, M.; Lin, C.S.; Brant, W.; Lue, T.F. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: Part I. J. Sex. Med. 2006, 3, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Bella, A.J.; Lue, T.F.; Lin, C.S. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: Part 2. J. Sex. Med. 2006, 3, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.S.; Bochinski, D.J.; Lin, G.T.; Nunes, L.; Lin, C.S.; Lue, T.F. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int. 2003, 92, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Bakircioglu, M.E.; Lin, C.S.; Fan, P.; Sievert, K.D.; Kan, Y.W.; Lue, T.F. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J. Urol. 2001, 165, 2103–2109. [Google Scholar] [CrossRef]
- Harrisberger, F.; Smieskova, R.; Schmidt, A.; Lenz, C.; Walter, A.; Wittfeld, K.; Grabe, H.J.; Lang, U.E.; Fusar-Poli, P.; Borgwardt, S. BDNF Val66Met polymorphism and hippocampal volume in neuropsychiatric disorders: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2015, 55, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, G.; Pettorruso, M.; de Berardis, D.; Varasano, P.A.; Lucidi Pressanti, G.; de Remigis, V.; Valchera, A.; Ricci, V.; di Nicola, M.; Janiri, L.; Biggio, G.; Di Giannantonio, M. Agomelatine increases BDNF serum levels in depressed patients in correlation with the improvement of depressive symptoms. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, G.; di Iorio, G.; Marini, S.; Ricci, V.; de Berardis, D.; di Giannantonio, M. Nerve growth factor and brain-derived neurotrophic factor concentrations in schizophrenia: A review. J. Biol. Regul. Homeost. Agents 2012, 26, 347–356. [Google Scholar] [PubMed]
- Campos, C.; Rocha, N.B.; Nardi, A.E.; Lattari, E.; Machado, S. Exercise induced neuroplasticity to enhance therapeutic outcomes of cognitive remediation in Schizophrenia: Analyzing the role of brain-derived neurotrophic factor. CNS Neurol. Disord. Drug Targets 2016. [Google Scholar] [CrossRef]
- Nuernberg, G.L.; Aguiar, B.; Bristot, G.; Fleck, M.P.; Rocha, N.S. Brain-derived neurotrophic factor increase during treatment in severe mental illness inpatients. Transl. Psychiatry 2016, 6, e985. [Google Scholar] [CrossRef] [PubMed]
- Tep, C.; Kim, M.L.; Opincariu, L.I.; Limpert, A.S.; Chan, J.R.; Appel, B.; Carter, B.D.; Yoon, S.O. Brain-derived neurotrophic factor (BDNF) induces polarized signaling of small GTPase (Rac1) protein at the onset of Schwann cell myelination through partitioning-defective 3 (Par3) protein. J. Biol. Chem. 2012, 287, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- May, F.; Matiasek, K.; Vroemen, M.; Caspers, C.; Mrva, T.; Arndt, C.; Schlenker, B.; Gais, P.; Brill, T.; Buchner, A.; et al. GDNF-transduced Schwann cell grafts enhance regeneration of erectile nerves. Eur. Urol. 2008, 54, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Al-Abbad, H.; Simon, J.V. The effectiveness of extracorporeal shock wave therapy on chronic achilles tendinopathy: A systematic review. Foot Ankle Int. 2013, 34, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Fukumoto, Y.; Shimokawa, H. Extracorporeal shock wave therapy for ischemic cardiovascular disorders. Am. J. Cardiovasc. Drugs 2011, 11, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, I.; Appel, B.; Vardi, Y. Low-intensity extracorporeal shock wave therapy—A novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy. J. Sex. Med. 2012, 9, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Inoue, G.; Mannoji, C.; Saisu, T.; Takahashi, K.; Mitsuhashi, S.; Wada, Y.; Takahashi, K.; Yamagata, M.; Moriya, H. Shock wave application to rat skin induces degeneration and reinnervation of sensory nerve fibres. Neurosci. Lett. 2001, 315, 57–60. [Google Scholar] [CrossRef]
- Murata, R.; Ohtori, S.; Ochiai, N.; Takahashi, N.; Saisu, T.; Moriya, H.; Takahashi, K.; Wada, Y. Extracorporeal shockwaves induce the expression of ATF3 and GAP-43 in rat dorsal root ganglion neurons. Auton Neurosci. 2006, 128, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Lin, G.; Xin, Z.; Ferretti, L.; Zhang, H.; Lue, T.F.; Lin, C.S. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J. Sex. Med. 2013, 10, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Liu, J.; Li, H.; Wang, L.; Xu, Y.; Tian, W.; Lin, G.; Xin, Z. Low-intensity shock wave therapy and its application to erectile dysfunction. World J. Mens. Health 2013, 31, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Matheu, M.P.; Sun, F.; Wang, L.; Sanford, M.T.; Ning, H.; Banie, L.; Lee, Y.C.; Xin, Z.; Guo, Y.; et al. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. J. Sex. Med. 2016, 13, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Kim, H.; Yoon, H. ER stress-mediated signaling: Action potential and Ca2+ as key players. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Novoa, I.; Zeng, H.; Harding, H.P.; Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell. Biol. 2001, 153, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, J.O.; Laurikainen, A.; Klinge, E.; Saarma, M. Neurotrophin-3 is a target-derived neurotrophic factor for penile erection-inducing neurons. Neuroscience 2005, 133, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.C.; Wu, S.H. Neurotrophin signaling: Many exciting surprises! Cell Mol. Life Sci. 2006, 63, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Willnow, T.E.; Petersen, C.M. p75NTR—Live or let die. Curr. Opin. Neurobiol. 2005, 15, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Okugawa, Y.; Toiyama, Y.; Inoue, Y.; Saigusa, S.; Kawamura, M.; Araki, T.; Uchida, K.; Mohri, Y.; Kusunoki, M. Brain-derived neurotrophic factor (BDNF)-induced tropomyosin-related kinase B (Trk B) signaling is a potential therapeutic target for peritoneal carcinomatosis arising from colorectal cancer. PLoS ONE 2014, 9, e96410. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.C.; Chiu, T.H.; Huang, Y.T. Overexpression of BDNF and TrkB in human bladder cancer specimens. Oncol. Rep. 2010, 24, 1265–1270. [Google Scholar] [PubMed]
- Okamura, K.; Harada, T.; Wang, S.; Ijichi, K.; Furuyama, K.; Koga, T.; Okamoto, T.; Takayama, K.; Yano, T.; Nakanishi, Y. Expression of TrkB and BDNF is associated with poor prognosis in non-small cell lung cancer. Lung Cancer 2012, 78, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, S.; Meignan, S.; Lagadec, C.; Germain, E.; Hondermarck, H.; Adriaenssens, E.; Le Bourhis, X. Overexpression of p75(NTR) increases survival of breast cancer cells through p21(WAF1). Cell Signal. 2010, 22, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Menei, P.; Montero-Menei, C.; Whittemore, S.R.; Bunge, R.P.; Bunge, M.B. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur. J. Neurosci. 1998, 10, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lin, G.; Reed-Maldonado, A.; Wang, C.; Lee, Y.C.; Lue, T.F. Low-intensity extracorporeal shock wave treatment improves erectile function: A systematic review and meta-analysis. Eur. Urol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Ito, K.; Hao, K.; Shindo, T.; Ogata, T.; Kagaya, Y.; Kurosawa, R.; Nishimiya, K.; Satoh, K.; Miyata, S.; et al. Extracorporeal low-energy shock-wave therapy exerts anti-inflammatory effects in a rat model of acute myocardial infarction. Circ. J. 2014, 78, 2915–2925. [Google Scholar] [CrossRef] [PubMed]
- Goertz, O.; Lauer, H.; Hirsch, T.; Ring, A.; Lehnhardt, M.; Langer, S.; Steinau, H.U.; Hauser, J. Extracorporeal shock waves improve angiogenesis after full thickness burn. Burns 2012, 38, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Tara, S.; Miyamoto, M.; Takagi, G.; Kirinoki-Ichikawa, S.; Tezuka, A.; Hada, T.; Takagi, I. Low-energy extracorporeal shock wave therapy improves microcirculation blood flow of ischemic limbs in patients with peripheral arterial disease: Pilot study. J. Nippon. Med. Sch. 2014, 81, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ioppolo, F.; Rompe, J.D.; Furia, J.P.; Cacchio, A. Clinical application of shock wave therapy (SWT) in musculoskeletal disorders. Eur. J. Phys. Rehabil. Med. 2014, 50, 217–230. [Google Scholar] [PubMed]
- Lin, G.; Zhang, H.; Sun, F.; Lu, Z.; Reed-Maldonado, A.; Lee, Y.C.; Wang, G.; Banie, L.; Lue, T.F. Brain-derived neurotrophic factor promotes nerve regeneration by activating the JAK/STAT pathway in Schwann cells. Transl. Androl. Urol. 2016, 5, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Weihs, A.M.; Fuchs, C.; Teuschl, A.H.; Hartinger, J.; Slezak, P.; Mittermayr, R.; Redl, H.; Junger, W.G.; Sitte, H.H.; Runzler, D. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J. Biol. Chem. 2014, 289, 27090–27104. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Axten, J.M.; Romeril, S.P.; Shu, A.; Ralph, J.; Medina, J.R.; Feng, Y.; Li, W.H.; Grant, S.W.; Heerding, D.A.; Minthorn, E.; et al. Discovery of GSK2656157: An optimized PERK inhibitor selected for preclinical development. ACS Med. Chem. Lett. 2013, 4, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Formenti, F.; Constantin-Teodosiu, D.; Emmanuel, Y.; Cheeseman, J.; Dorrington, K.L.; Edwards, L.M.; Humphreys, S.M.; Lappin, T.R.; McMullin, M.F.; McNamara, C.J.; et al. Regulation of human metabolism by hypoxia-inducible factor. Proc. Natl. Acad. Sci. USA 2010, 107, 12722–12727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albersen, M.; Fandel, T.M.; Lin, G.; Wang, G.; Banie, L.; Lin, C.S.; Lue, T.F. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J. Sex. Med. 2010, 7, 3331–3340. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence of Primers (5′–3′) |
|---|---|
| BDNF | Forward: GATGCTCAGCAGTCAAGTGCCTTT |
| Reverse: AGAAAGAGCAGAGGAGGCTCCAAA | |
| ATF4 | Forward: ATGGCTGGCTATGGATGG |
| Reverse: GGGAAGAGGCTGCAAGAA | |
| HIF1α | Forward: CATCTCCACCTTCTACCC |
| Reverse: TCCAAGAAAGCGACATAG | |
| GDNF | Forward: CCAGAGAATTCCAGAGGGAAAG |
| Reverse: TCAGATACATCCACACCGTTTAG | |
| β-Actin | Forward: CTACAATGAGCTGCGTGTG |
| Reverse: AATGTCACGCACGATTTCCC | |
| siATF4 | Forward: GCCATCTCCCAGAAAGTGTAATA |
| Reverse: GTCATAAGGTTTGGGTCGAGAA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Ning, H.; Reed-Maldonado, A.B.; Zhou, J.; Ruan, Y.; Zhou, T.; Wang, H.S.; Oh, B.S.; Banie, L.; Lin, G.; et al. Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 433. https://doi.org/10.3390/ijms18020433
Wang B, Ning H, Reed-Maldonado AB, Zhou J, Ruan Y, Zhou T, Wang HS, Oh BS, Banie L, Lin G, et al. Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signaling Pathway. International Journal of Molecular Sciences. 2017; 18(2):433. https://doi.org/10.3390/ijms18020433
Chicago/Turabian StyleWang, Bohan, Hongxiu Ning, Amanda B. Reed-Maldonado, Jun Zhou, Yajun Ruan, Tie Zhou, Hsun Shuan Wang, Byung Seok Oh, Lia Banie, Guiting Lin, and et al. 2017. "Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signaling Pathway" International Journal of Molecular Sciences 18, no. 2: 433. https://doi.org/10.3390/ijms18020433






