Redistribution of Cerebral Blood Flow during Severe Hypovolemia and Reperfusion in a Sheep Model: Critical Role of α1-Adrenergic Signaling
Abstract
:1. Introduction
2. Results
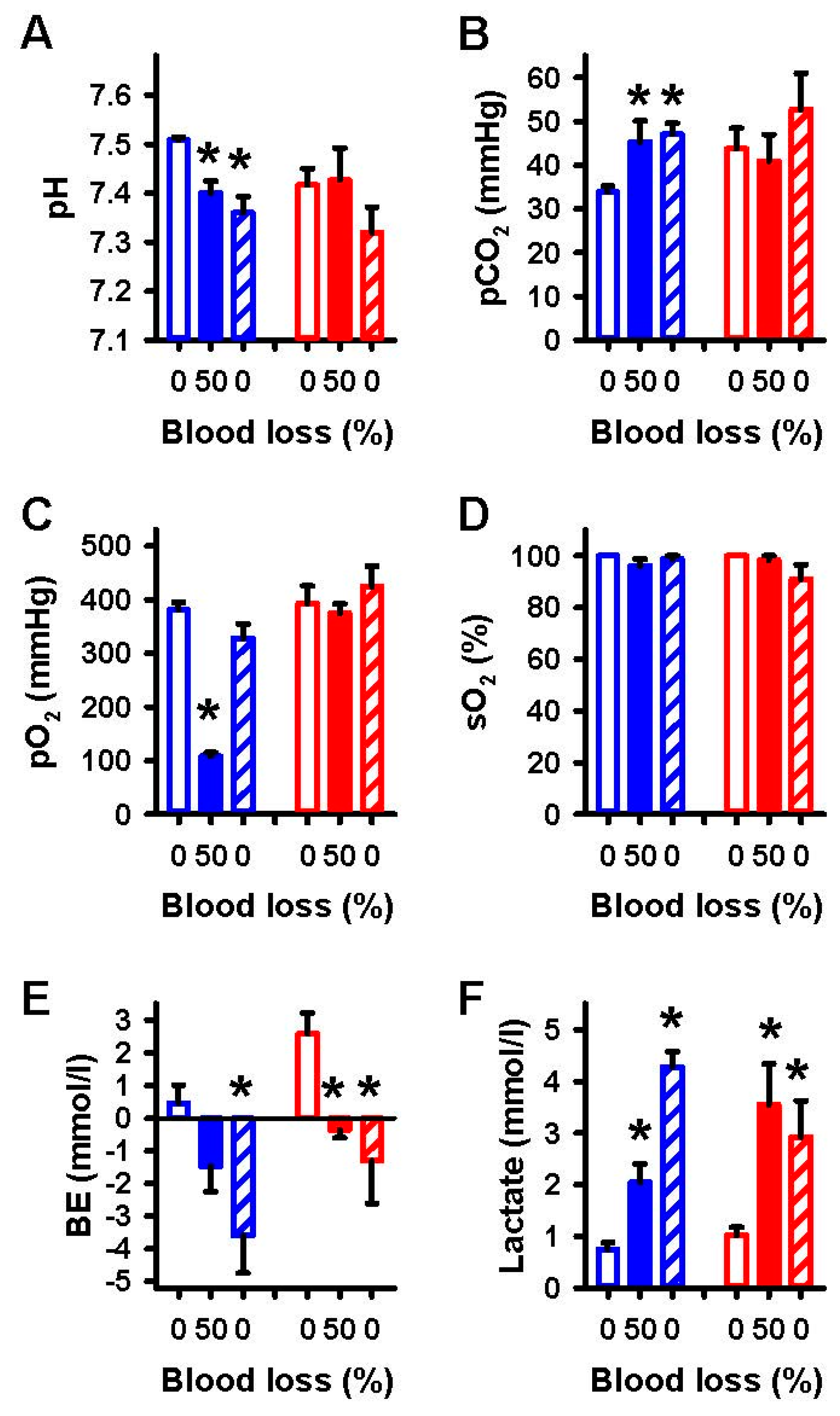
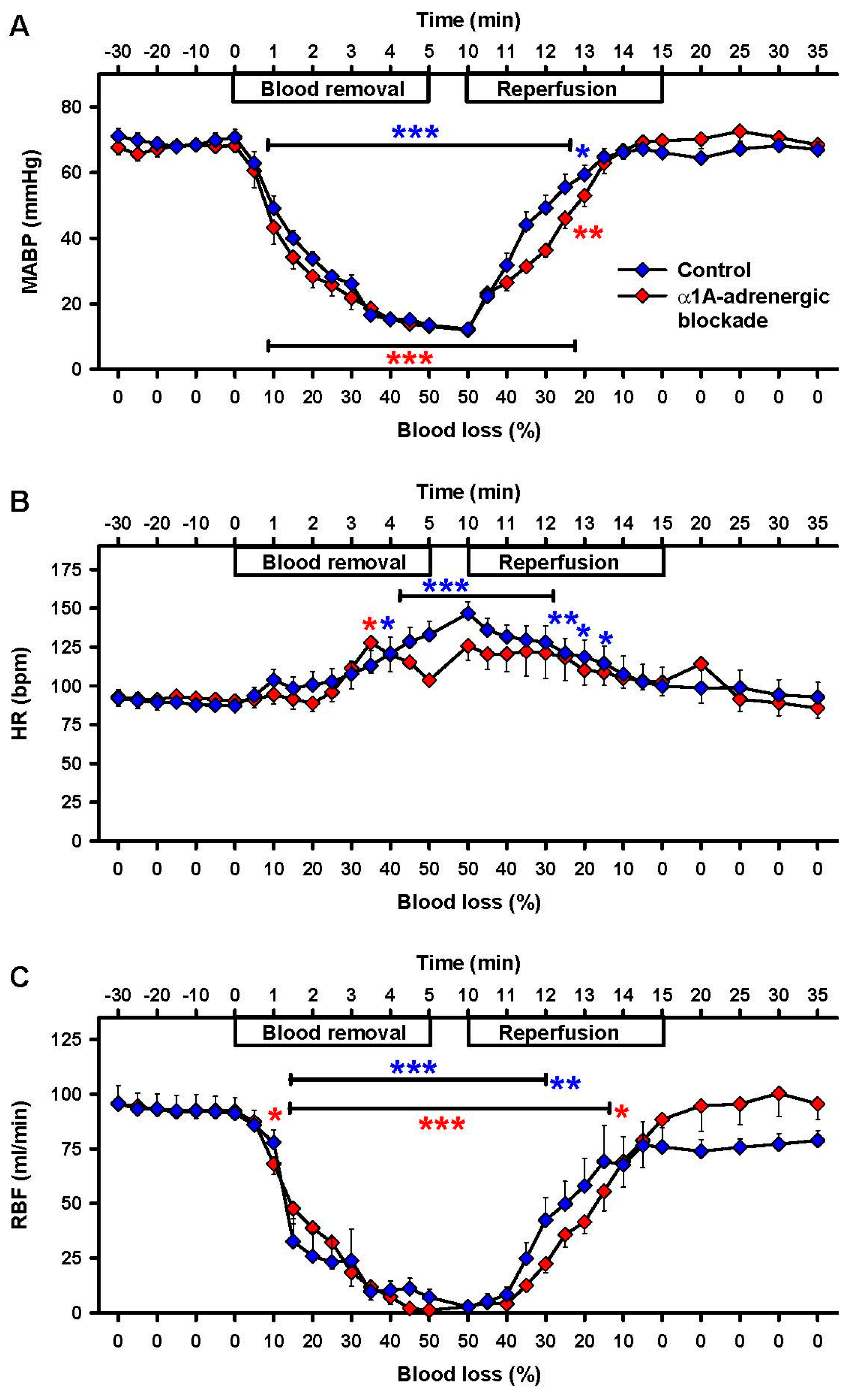
2.1. Effects of Severe Hemorrhage on Arterial Blood Gases and Vital Parameters
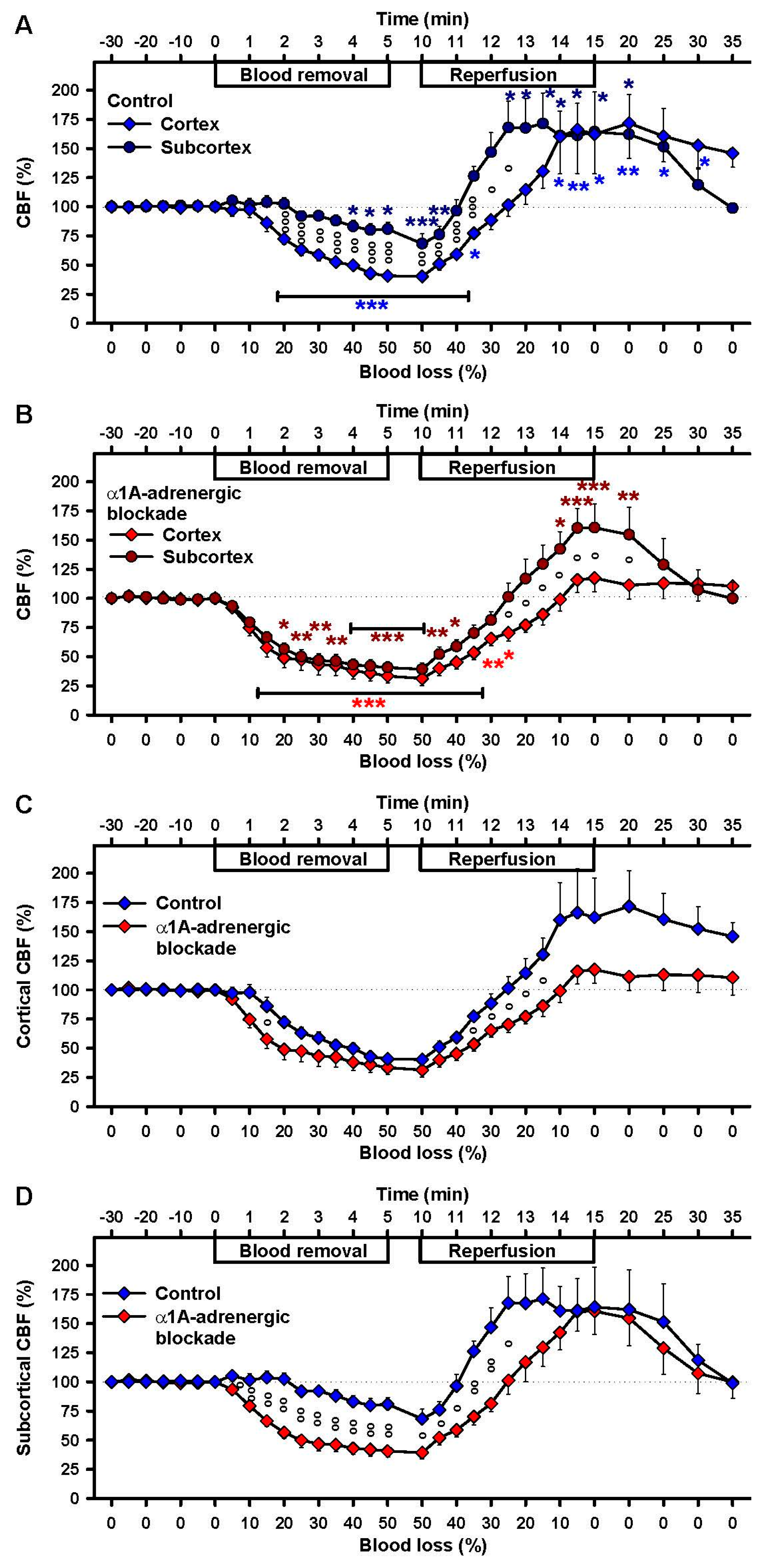
2.2. Effects of Severe Hemorrhage on CBF
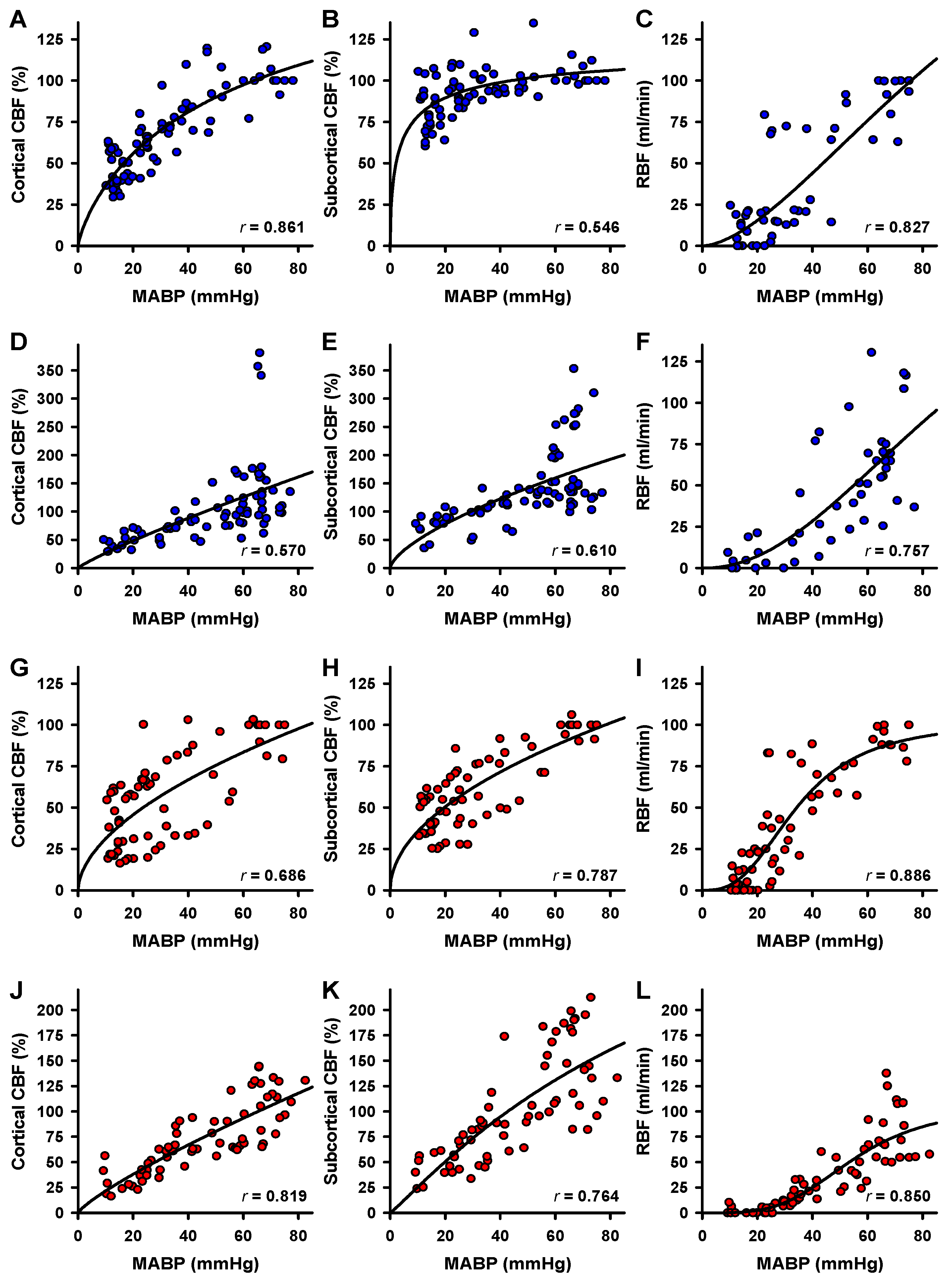
2.3. Correlation of Blood Flow and MABP
2.4. Effects of α1A-Adrenergic Blockade
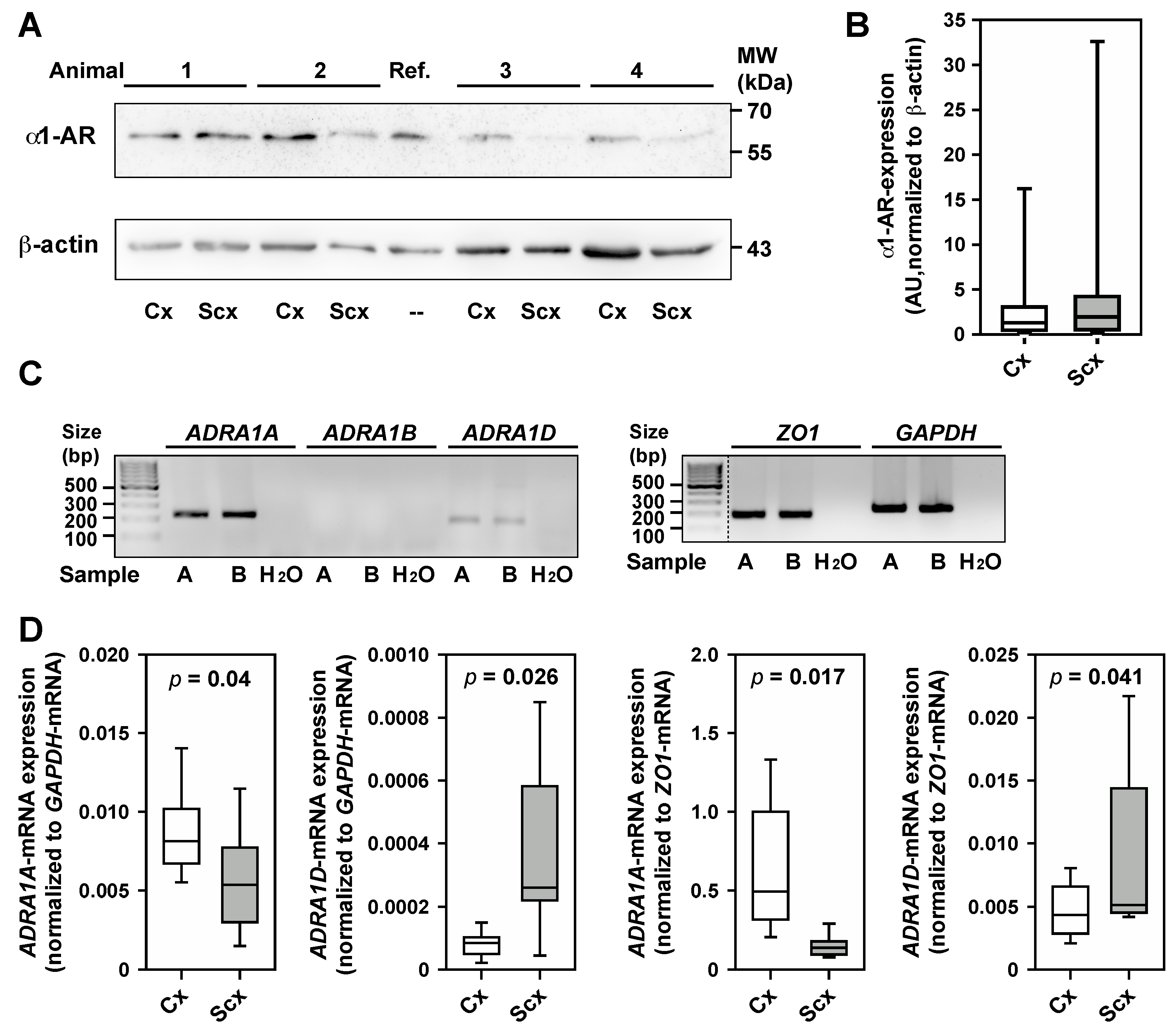
2.5. Expression of α1-Adrenergic Receptors
3. Discussion
3.1. Clinical Implications
3.2. Potential Roles of α1-Adrenergic Receptors
4. Materials and Methods
4.1. Surgical Instrumentation
4.2. Hypovolemia and Reperfusion
4.3. α1A-Adrenergic Blockade
4.4. Sample Preparation and Western Blotting
4.5. Quantitative RT-PCR
4.6. Data Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| α1-AR | α1-adrenergic receptor (at the protein level) |
| CBF | cerebral blood flow |
| ECG | electrocardiogram |
| HR | heart rate |
| MABP | mean arterial blood pressure |
| qRT-PCR | quantitative real-time polymerase chain-reaction |
| RBF | renal blood flow |
| ADRA1A (NC_019459.2) | sheep gene for adrenoceptor alpha 1A (GenBank accession number) |
| ADRA1B (NC_019462.2) | sheep gene for adrenoceptor alpha 1B (GenBank accession number) |
| ADRA1D (NC_019470.2) | sheep gene for adrenoceptor alpha 1D (GenBank accession number) |
| GAPDH (NC_019460.2) | sheep gene for glyceraldehyde-3-phosphate dehydrogenase (GenBank accession number) |
| ZO1 (NC_019475.2) | sheep gene for tight junction protein 1 (GenBank accession number) |
References
- Kauvar, D.S.; Lefering, R.; Wade, C.E. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J. Trauma 2006, 60, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Gutierrez, G.; Reines, H.D.; Wulf-Gutierrez, M.E. Clinical review: Hemorrhagic shock. Crit. Care 2004, 8, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Schiffner, R.; Antonow-Schlorke, I.; Mueller, T.; Frasch, M.; Rupprecht, S.; Schubert, H.; Schwab, M. Potential importance of alpha-receptors for distribution of cerebral blood flow (CBF) during umbilical cord occlusion (UCO) in fetal sheep-effects of betamethasone (BM). Reprod. Sci. 2010, 17, 300. [Google Scholar]
- Shevell, T.; Malone, F.D. Management of obstetric hemorrhage. Semin. Perinatol. 2003, 27, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Ohtaki, H.; Dohi, K.; Yin, L.; Nakamachi, T.; Endo, S.; Yofu, S.; Hiraizumi, Y.; Miyaoka, H.; Shioda, S. Neuronal damage in rat brain and spinal cord after cardiac arrest and massive hemorrhagic shock. Crit. Care Med. 2006, 34, 2820–2826. [Google Scholar] [CrossRef] [PubMed]
- Heckbert, S.R.; Vedder, N.B.; Hoffman, W.; Winn, R.K.; Hudson, L.D.; Jurkovich, G.J.; Copass, M.K.; Harlan, J.M.; Rice, C.L.; Maier, R.V. Outcome after hemorrhagic shock in trauma patients. J. Trauma 1998, 45, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.; Chesnut, R.M. Static autoregulation is intact in majority of patients with severe traumatic brain injury. J. Trauma 2009, 67, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Thebaud, B.; Husson, B.; Navelet, Y.; Huault, G.; Landrieu, P.; Devictor, D.; Sebire, G. Haemorrhagic shock and encephalopathy syndrome: Neurological course and predictors of outcome. Intensive Care Med. 1999, 25, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Pegram, B.L.; Kobrin, I.; Natsume, T.; Gallo, A.J.; Frohlich, E.D. Systemic and regional hemodynamic effects of acute and prolonged treatment with urapidil or prazosin in normotensive and spontaneously hypertensive rats. Am. J. Med. 1984, 77, 64–73. [Google Scholar] [CrossRef]
- Puchstein, C. Treatment of hypertensive crises in neurosurgery. Ann. Fr. Anesth. Reanim. 1989, 8, 598–602. [Google Scholar] [CrossRef]
- Kick, O.; van Aken, H.; Wouters, P.F.; Verbesselt, K.; van Hemelrijck, J. Vital organ blood flow during deliberate hypotension in dogs. Anesth. Analg. 1993, 77, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Waschke, K.F.; Riedel, M.; Lenz, C.; Albrecht, D.M.; van Ackern, K.; Kuschinsky, W. Regional heterogeneity of cerebral blood flow response to graded pressure-controlled hemorrhage. J. Trauma 2004, 56, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Hoyer, D.; Walter, B.; Gaser, E.; Kluge, H.; Zwiener, U. Changed systemic and cerebral hemodynamics and oxygen supply due to gradual hemorrhagic hypotension induced by an external PID-controller in newborn swine. Exp. Toxicol. Pathol. 1997, 49, 469–476. [Google Scholar] [CrossRef]
- DeWitt, D.S.; Prough, D.S.; Taylor, C.L.; Whitley, J.M.; Deal, D.D.; Vines, S.M. Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am. J. Physiol. 1992, 263, 1276–1284. [Google Scholar]
- Sadoshima, S.; Heistad, D.D. Regional cerebral blood flow during hypotension in normotensive and stroke-prone spontaneously hypertensive rats: Effect of sympathetic denervation. Stroke J. Cereb. Circ. 1983, 14, 575–579. [Google Scholar] [CrossRef]
- Bond, R.F.; Johnson, G. Cardiovascular adrenoreceptor balance during hemorrhagic hypotension and shock. Circ. Shock 1985, 16, 155–164. [Google Scholar] [PubMed]
- Rosenblum, W.I.; Chen, M. Density of perivascular nerves on some cerebral and extracerebral blood-vessels. Blood Vessels 1976, 13, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Cleveland, J.C.; Meng, X.Z.; Sheridan, B.C.; Banerjee, A.; Harken, A.H. Hemorrhage induces acute cardioadaptation to ischemia-reperfusion by an α1-adrenoceptor-mediated, protein synthesis-independent mechanism. Am. J. Physiol. 1997, 272, 718–725. [Google Scholar]
- Ogoh, S.; Hirasawa, A.; Raven, P.B.; Rebuffat, T.; Denise, P.; Lericollais, R.; Sugawara, J.; Normand, H. Effect of an acute increase in central blood volume on cerebral hemodynamics. Am. J. Physiol. 2015, 309, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Hirasawa, A.; Sugawara, J.; Nakahara, H.; Ueda, S.; Shoemaker, J.K.; Miyamoto, T. The effect of an acute increase in central blood volume on the response of cerebral blood flow to acute hypotension. J. Appl. Physiol. 2015, 119, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.Z.; Hou, W.G.; Chui, J.; Han, R.Q.; Gelb, A.W. Cardiac output and cerebral blood flow. Anesthesiology 2015, 123, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Toth, P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J. Vasc. Res. 2012, 49, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Takizawa, S.; Takagi, S.; Shinohara, Y. Mild hypothermia disturbs regional cerebrovascular autoregulation in awake rats. Brain Res. 1998, 789, 68–73. [Google Scholar] [CrossRef]
- Tuor, U.I.; Grewal, D. Autoregulation of cerebral blood flow: Influence of local brain development and postnatal age. Am. J. Physiol. 1994, 267, 2220–2228. [Google Scholar]
- Mueller, S.M.; Heistad, D.D.; Marcus, M.L. Total and regional cerebral blood flow during hypotension, hypertension, and hypocapnia. Effect of sympathetic denervation in dogs. Circ. Res. 1977, 41, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Komjati, K.; Sandor, P.; Reivich, M.; Greenberg, J.H.; Kovach, A.G.; Jaggi, J.L.; Nyary, I. Regional heterogeneity and differential vulnerability of cerebral and spinal vascular CO2-responsiveness during graded haemorrhagic hypotension. Acta Physiol. Hung. 1996, 84, 229–249. [Google Scholar] [PubMed]
- Dirnagl, U.; Kaplan, B.; Jacewicz, M.; Pulsinelli, W. Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. J. Cereb. Blood Flow Metab. 1989, 9, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, M.; Lauritzen, M. Laser-Doppler evaluation of rat brain microcirculation: Comparison with the [14C]-iodoantipyrine method suggests discordance during cerebral blood flow increases. J. Cereb. Blood Flow Metab. 1996, 16, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.J.; Schmidt, M.; Lehmann, T.; Irintchev, A.; Schubert, H.; Jung, C.; Schwab, M.; Huber, O.; Matziolis, G.; Schiffner, R. Increase of cortical cerebral blood flow and further cerebral microcirculatory effects of Serelaxin in a sheep model. Am. J. Physiol. 2016, 311, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Fisher, J.P.; Seifert, T.; Overgaard, M.; Secher, N.; Ogoh, S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp. Physiol. 2012, 97, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Garnett, A.R.; Glauser, F.L.; Ornato, J.P. Hypercarbic arterial acidemia following resuscitation from severe hemorrhagic shock. Resuscitation 1989, 17, 55–61. [Google Scholar] [CrossRef]
- Mccalden, T.A. Sympathetic control of the cerebral-circulation. J. Auton. Pharmacol. 1981, 1, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Minneman, K.P. Recent progress in α1-adrenergic receptor research. Acta Pharmacol. Sin. 2005, 26, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Piascik, M.T.; Perez, D.M. α1-adrenergic receptors: New insights and directions. J. Pharmacol. Exp. Ther. 2001, 298, 403–410. [Google Scholar] [PubMed]
- Hein, P.; Michel, M.C. Signal transduction and regulation: Are all α1-adrenergic receptor subtypes created equal? Biochem. Pharmacol. 2007, 73, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, T.; Yamauchi, J.; Hirasawa, A.; Tanoue, A.; Tsujimoto, G. Recent progress in α1-adrenoceptor pharmacology. Biol. Pharm. Bull 2002, 25, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Mittal, A.; Chu, N.N.; Zhang, L.B.; Longo, L.D. α1-Adrenergic receptor subtype function in fetal and adult cerebral arteries. Am. J. Physiol. 2010, 298, 1797–1806. [Google Scholar]
- Buch, J. Urapidil, a dual-acting antihypertensive agent: Current usage considerations. Adv. Ther. 2010, 27, 426–443. [Google Scholar] [CrossRef] [PubMed]
- Rokosh, D.G.; Simpson, P.C. Knockout of the α1A/C-adrenergic receptor subtype: The α1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc. Natl. Acad. Sci. USA 2002, 99, 9474–9479. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, M.; Verge, D.; Gozlan, H.; Pichat, L.; Hamon, M. Autoradiographic evidence for the heterogeneity of 5-HT1 sites in the rat brain. Brain Res. 1984, 291, 159–163. [Google Scholar] [CrossRef]
- Lanfumey, L.; Hamon, M. Central 5-HT1A receptors: Regional distribution and functional characteristics. Nucl. Med. Biol. 2000, 27, 429–435. [Google Scholar] [CrossRef]
- Barnes, J.M.; Costall, B.; Coughlan, J.; Domeney, A.M.; Gerrard, P.A.; Kelly, M.E.; Naylor, R.J.; Onaivi, E.S.; Tomkins, D.M.; Tyers, M.B. The effects of ondansetron, a 5-HT3 receptor antagonist, on cognition in rodents and primates. Pharmacol. Biochem. Behav. 1990, 35, 955–962. [Google Scholar] [CrossRef]
- De Andrade, C.R.; Fukada, S.Y.; Olivon, V.C.; de Godoy, M.A.; Haddad, R.; Eberlin, M.N.; Cunha, F.Q.; de Souza, H.P.; Laurindo, F.R.; de Oliveira, A.M. α1D-adrenoceptor-induced relaxation on rat carotid artery is impaired during the endothelial dysfunction evoked in the early stages of hyperhomocysteinemia. Eur. J. Pharmacol. 2006, 543, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Pernomian, L.; Gomes, M.S.; Restini, C.B.; Pupo, A.S.; de Oliveira, A.M. Cross-talk with β2-adrenoceptors enhances ligand affinity properties from endothelial α1D-adrenoceptors that mediates carotid relaxation. J. Pharm. Pharmacol. 2013, 65, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Yang, M.; Goepel, M.; Michel, M.C. Comparison of human α1-adrenoceptor subtype coupling to protein kinase C activation and related signalling pathways. NS Arch. Pharmacol. 1998, 357, 100–110. [Google Scholar] [CrossRef]
- Keffel, S.; Alexandrov, A.; Goepel, M.; Michel, M.C. α1-Adrenoceptor subtypes differentially couple to growth promotion and inhibition in Chinese hamster ovary cells. Biochem. Biophys. Res. Commun. 2000, 272, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Institute of Laboratory Animal Resources (U.S.); Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. In NIH Publication; U.S. Department of Health and Human Services, Public Health Service: Bethesda, MD, USA, 2011. [Google Scholar]
- Muller, T.; Lohle, M.; Schubert, H.; Bauer, R.; Wicher, C.; Antonow-Schlorke, I.; Sliwka, U.; Nathanielsz, P.W.; Schwab, M. Developmental changes in cerebral autoregulatory capacity in the fetal sheep parietal cortex. J. Physiol. 2002, 539, 957–967. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiffner, R.; Bischoff, S.J.; Lehmann, T.; Rakers, F.; Rupprecht, S.; Reiche, J.; Matziolis, G.; Schubert, H.; Schwab, M.; Huber, O.; et al. Redistribution of Cerebral Blood Flow during Severe Hypovolemia and Reperfusion in a Sheep Model: Critical Role of α1-Adrenergic Signaling. Int. J. Mol. Sci. 2017, 18, 1031. https://doi.org/10.3390/ijms18051031
Schiffner R, Bischoff SJ, Lehmann T, Rakers F, Rupprecht S, Reiche J, Matziolis G, Schubert H, Schwab M, Huber O, et al. Redistribution of Cerebral Blood Flow during Severe Hypovolemia and Reperfusion in a Sheep Model: Critical Role of α1-Adrenergic Signaling. International Journal of Molecular Sciences. 2017; 18(5):1031. https://doi.org/10.3390/ijms18051031
Chicago/Turabian StyleSchiffner, René, Sabine Juliane Bischoff, Thomas Lehmann, Florian Rakers, Sven Rupprecht, Juliane Reiche, Georg Matziolis, Harald Schubert, Matthias Schwab, Otmar Huber, and et al. 2017. "Redistribution of Cerebral Blood Flow during Severe Hypovolemia and Reperfusion in a Sheep Model: Critical Role of α1-Adrenergic Signaling" International Journal of Molecular Sciences 18, no. 5: 1031. https://doi.org/10.3390/ijms18051031





