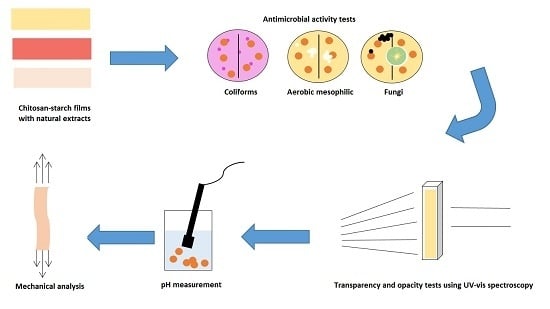
Antimicrobial, Optical and Mechanical Properties of Chitosan–Starch Films with Natural Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antimicrobial Activity
2.1.1. Previous Contamination Test
2.1.2. Activity against Aerobic Mesophilic Bacteria
2.1.3. Activity against Coliforms
2.1.4. Activity against Fungi
2.2. Thickness Measurement
2.3. Optical Properties (Transparency and Opacity)
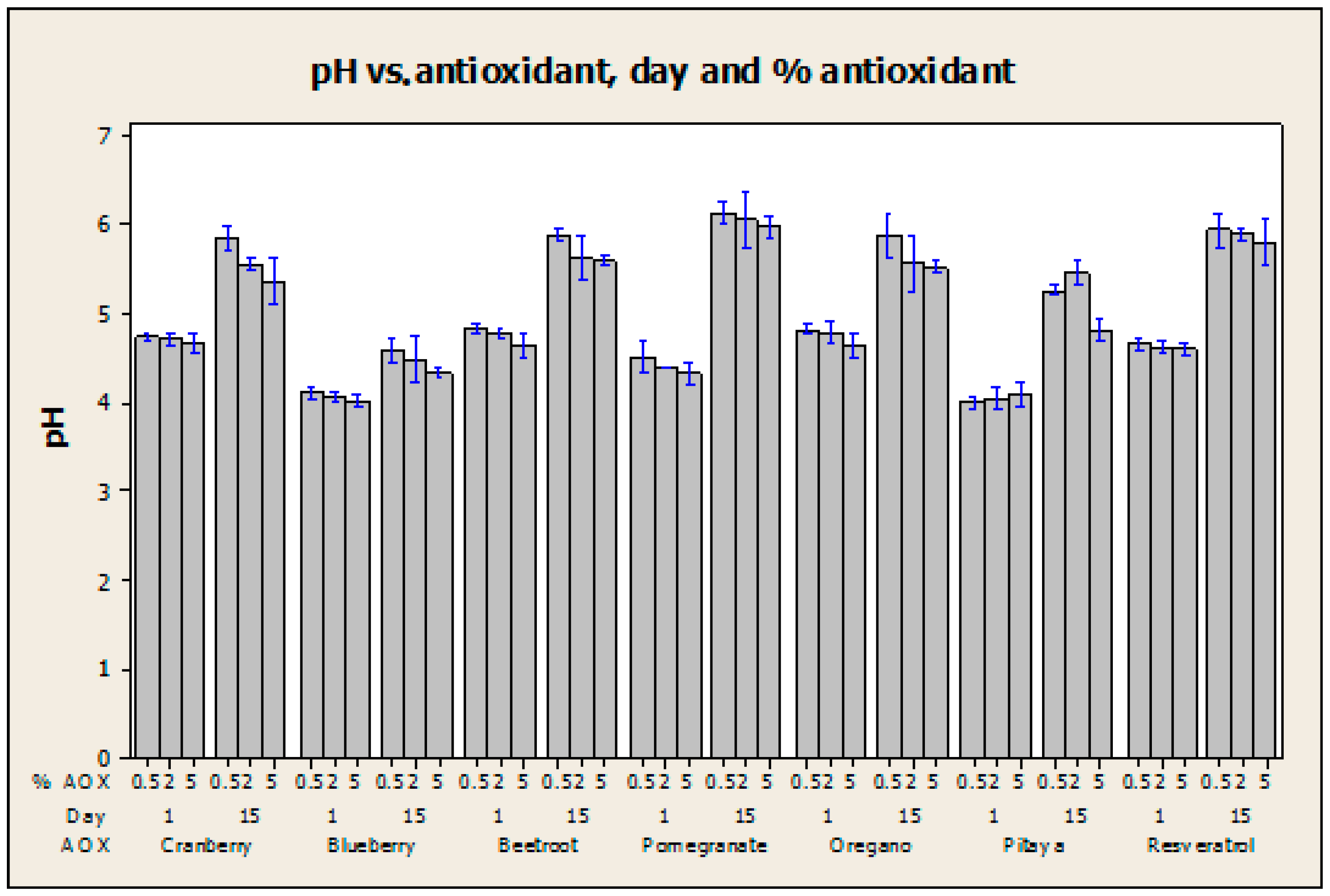
2.4. pH Measurement
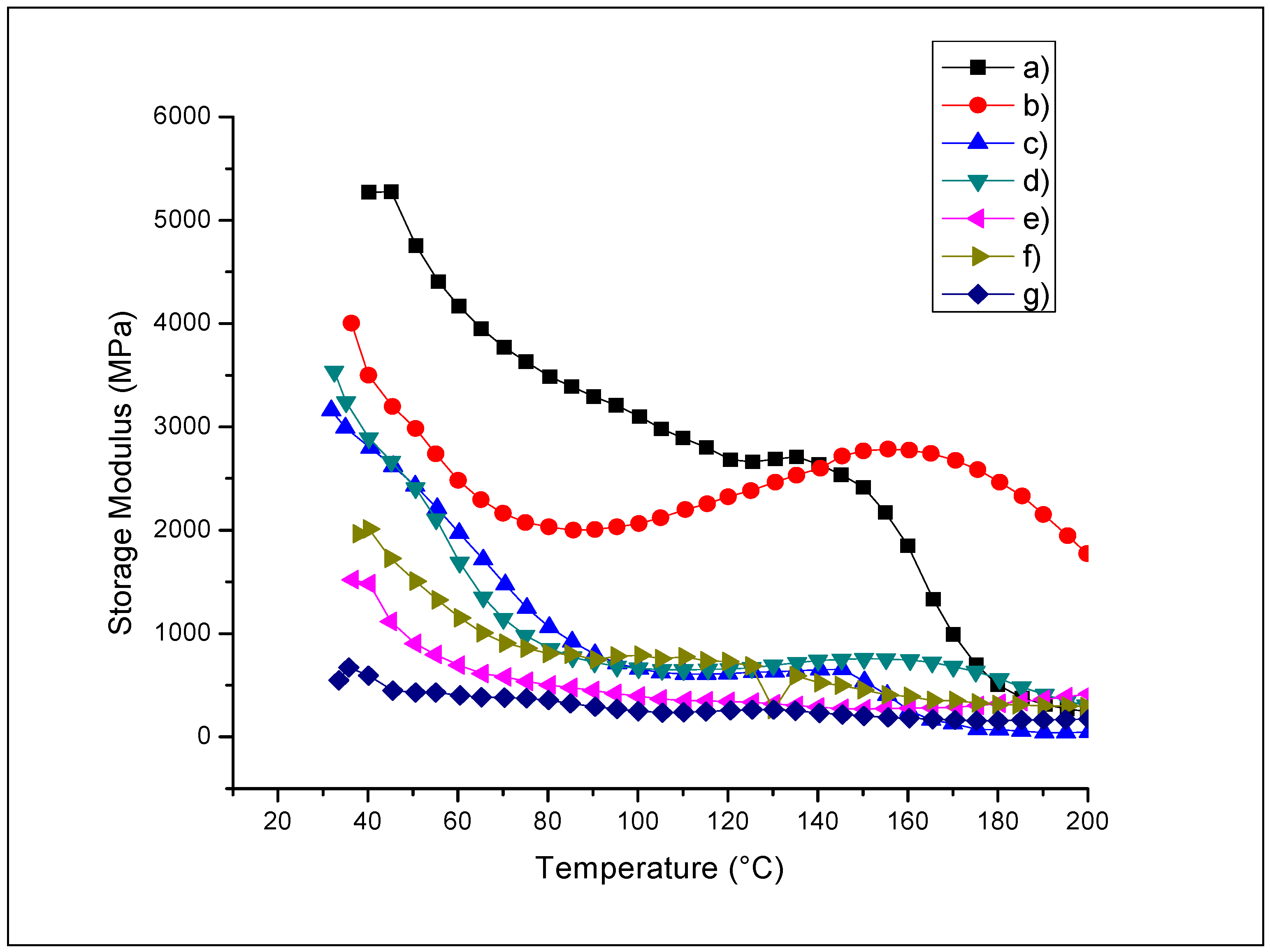
2.5. Dynamic Mechanic Analysis (DMA)
2.5.1. Storage Modulus (E’)
2.5.2. Glass Transition Temperature (Tg)
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of Films
3.3. Antimicrobial Activity
3.3.1. Previous Contamination Test
3.3.2. Activity against Aerobic Mesophilic Bacteria
3.3.3. Activity against Coliforms
3.3.4. Activity against Fungi
3.4. Thickness Measurement
3.5. Optical Properties Using Ultraviolet–Visible Spectroscopy
3.6. pH Measurement
3.7. Dynamic Mechanic Analysis (DMA)
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ai, H.; Wangb, F.; Xia, Y.; Chen, X.; Lei, C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012, 132, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Nguyen, V.Q.; Mori, Y.; Nakamura, S.; Hattori, H. Adsorption of Silver Nanoparticles onto Different Surface Structures of Chitin/Chitosan and Correlations with Antimicrobial Activities. Int. J. Mol. Sci. 2015, 16, 13973–13988. [Google Scholar] [CrossRef] [PubMed]
- Xua, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–starch composite film: Preparation and characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, R.; Ríos, L.A.; Betancur, L.A.; Ocampo, D.M. Curso Práctico de Química Orgánica Enfocado a Biología y Alimentos, 1st ed.; Editorial Universidad de Caldas: Manizales, Colombia, 2008. [Google Scholar]
- Marañón-Ruiz, V.F.; Rizo de la Torre, L.D.C. Caracterización de las propiedades ópticas de Betacianinas y Betaxantinas por espectroscopía UV–VIS y barrido en Z. Superficies y Vacío 2011, 24, 113–120. [Google Scholar]
- García-García, R.M.; Palou-García, E. Mecanismos de acción antimicrobiana de timol y carvacrol sobre microorganismos de interés en alimentos. Temas Selectos de Ingeniería de Alimentos 2008, 2, 41–51. [Google Scholar]
- Gambini, J.; López-Grueso, R.; Olaso-González, G.; Inglés, M.; Abdelazida, K.; El-Alami, M.; Bonet-Costa, V. Borrás, C.; Viña, J. Resveratrol: Distribución, propiedades y perspectivas. Revista Española de Geriatría y Gerontología 2013, 48, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, F.S.; Clark, D.S. Análisis Microbiológico de los Alimentos, 1st ed.; Acribia: Zaragoza, Spain, 1973; pp. 31–33. [Google Scholar]
- Fatemeh, D.; Reza, Z.M.; Mohammad, A.; Salomeh, K.; Gholam Reza, A.; Hossein, S.; Maryam, S.; Azam, A.; Mana, S.; Negin, N.; et al. Rapid detection of coliforms in drinking water of Arak city using multiplex PCR method in comparison with the standard method of culture (Most Probably Number). Asian Pac. J. Trop. Biomed. 2014, 4, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, P.; Blengio Pinto, J.R. Microbiología, 2nd ed.; Nueva Editorial Interamericana S.A. de C.V.: Mexico City, Mexico, 1979; pp. 144–145, 150–157, 218, 334–335, 376. [Google Scholar]
- Walker, S. Microbiología, 4th ed.; McGraw-Hill: Mexico City, Mexico, 2000. [Google Scholar]
- Chakraborty, D.; Chakraborti, S. Bioassay-guided isolation and identification of antibacterial and antifungal component from methanolic extract of green tea leaves (Camellia Sinensis). Res. J. Phytochem. 2010, 4, 78–86. [Google Scholar] [CrossRef]
- Lárez Velásquez, C. Algunas potencialidades de la quitina y el quitosano para usos relacionados con la agricultura en Latinoamérica. Revista UDO Agrícola 2008, 8, 1–22. [Google Scholar]
- Lengeler, J.; Drews, G.; Schlege, H. Biology of the Prokaryotes, 2nd ed.; Thieme Publishing Group: Stuttgart, Germany, 1999. [Google Scholar]
- Barka, E.A.; Eullaffroy, P.; Clément, C.; Vernet, G. Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Van der Zee, R.; Koets, A.P.; De Graaff, A.M.; Van Knapen, F.; Gaastra, W.; Haagsman, H.; Veldhuizen, J.A. Carvacrol induces heat shock protein and inhibits synthesis of flagellin in Escherichia coli 0157: H7. Appl. Environ. Microb. 2007, 73, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana NOM-092-SSA1-1994, Bienes y Servicios. Método para la Cuenta de Bacterias Aerobias en placa, Diario Oficial de la Nación; Gobierno Constitucional de los Estados Unidos Mexicanos: Mexico City, Mexico, 1995. [Google Scholar]
- Norma Oficial Mexicana NOM-113-SSA1-1994, Bienes y Servicios. Método para la Cuenta de Microorganismos Coliformes Totales en Placa, Diario Oficial de la Nación; Gobierno Constitucional de los Estados Unidos Mexicanos: Mexico City, Mexico, 1995. [Google Scholar]
- Norma Oficial Mexicana NOM-111-SSA1-1994, Bienes y Servicios. Método para la Cuenta de Mohos y Levaduras en Alimentos, Diario Oficial de la Nación; Gobierno Constitucional de los Estados Unidos Mexicanos: Mexico City, Mexico, 1995. [Google Scholar]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of Antimicrobial Active Packaging ‘Containing Natamycin, Nisin, Pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Paparella, A.; Mazzarrino, G.; Chaves-Lopez, C.; Rossi, C.; Sacchetti, G.; Guerrieri, O.; Serio, A. Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016, 59, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.L.; Min Wu, J.; Chen, Y.; Zhao, G. Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll. 2010, 24, 285–290. [Google Scholar] [CrossRef]
- Pranoto, Y.; Rakshit, S.K.; Salokhe, V.M. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. Food Sci. Technol. 2005, 38, 859–865. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Plascencia-Jatomea, M.; Viniegra, G.; Olayo, R.; Castillo-Ortega, M.; Shirai, K. Effect of chitosan and temperature on spore germination of Aspergillus niger. Macromol. Biosci. 2003, 3, 582–586. [Google Scholar] [CrossRef]
- Ture, H.; Eroglu, E.; Ozen, B.; Soyer, F. Effect of biopolymers containing natamycin against Aspergillus niger and Penicillium roquefortii on fresh kashar cheese. Int. J. Food Sci. Technol. 2011, 46, 154–160. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Zhang, Z.-H.; Li, L.; Yuan, M.-L.; Fan, J.; Zhao, T.-R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Avena-Bustillos, R.J.; Du, W.-X.; Chiou, B.-S.; Williams, T.G.; Wood, D.; McHugh, T.H.; Soares, N.F.F. Physical and Antibacterial Properties of Acai Edible Films Formulated with Thyme Essential Oil and Apple Skin Polyphenols. J. Food Sci. 2014, 79, M903–M910. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Zupančič, S.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Jackman, R.L.; Smith, J.L. Anthocyanins and Betalains, in Natural Food Colorants, 2nd ed.; Blackie Academic & Professional: Londres, UK, 1996; pp. 244–309. [Google Scholar]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Gupta, D.; Singh, D.; Kothiyal, N.C.; Saini, A.K.; Singh, V.P.; Pathania, D. Synthesis of chitosan-g-poly(acrylamide)/ZnS nanocomposite for controlled drug delivery and antimicrobial activity. Int. J. Biol. Macromol. 2015, 74, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Del, R.; Moreira, M.; Pereda, M.; Marcovich, N.E.; Roura, S.I. Antimicrobial Effectiveness of Bioactive Packaging Materials from Edible Chitosan and Casein Polymers: Assessment on Carrot, Cheese, and Salami. J. Food Sci. 2011, 76, M54–M63. [Google Scholar]
- Al-Sagheer, F.; Merchant, S.S. Visco-elasctic properties of chitosan-titania nano-composites. Carbohydr. Polym. 2011, 85, 356–362. [Google Scholar] [CrossRef]
- Domínguez-Courtney, M.F.; Jiménez-Munguía, M.T. Películas formuladas con polisacaridos: Propiedades y aplicaciones. Temas Selectos de Ingeniería de Alimentos 2012, 6, 110–121. [Google Scholar]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Thermophysical properties of chitosan, chitosan–starch and chitosan-pullulan films near the glass transition. Carbohydr. Polym. 2002, 48, 179–190. [Google Scholar] [CrossRef]
- Torres-Delgado, C.L.; Diaz-Zavala, N.P.; Velasco-Santos, C.; Salas, P.; Martinez-Hernandez, A.L. Synthesis and Characterization of Chitosan–Starch Films Reinforced with TiO2 Nanoparticles; Memorias del XIX International Material Research Congress; Material Research Society & Sociedad Mexicana de Materiales A.C.: Cancun, Mexico, 2010. [Google Scholar]
- Parente, E.; Brienza, C.; Moles, M.; Ricciardi, A. A comparison of methods for the measurement of bacteriocin activity. J. Microbiol. Methods 1995, 22, 95–108. [Google Scholar] [CrossRef]
- Comité de Expertos en Antibióticos. Unificación de Métodos para las Pruebas de Sensibilidad Microbiana; Segundo Informe del Comité de Expertos en Antibióticos Serie de Informes Técnicos No 210; World Health Organization (WHO): Geneva, Switzerland, 1961. [Google Scholar]
- ASTM D 6739 Standard Test Method for Silica—pH Value, 2015. Available online: https://www.astm.org/Standards/D6739.htm (accessed on 22 August 2016).

| Code Sample | Aerobic Mesophilic Bacteria (CFU) | Coliforms (CFU) | Fungi (CFU) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Average Value | Test 1 | Test 2 | Average Value | Test 1 | Test 2 | Average Value | |
| QS2 | 1 | 4 | 2.5 ± 2.12 | <1 | 2 | 1 ± 1.41 | 10 | 3 | 6.5 ± 4.95 |
| QSA0.5 | <1 | <1 | <1 ± 0 | <1 | <1 | <1 ± 0 | <1 | <1 | <1 ± 0 |
| QSA2 | <1 | <1 | <1 ± 0 | <1 | <1 | <1 ± 0 | 1 | 1 | 1 ± 0 |
| QSA5 | 1 | 1 | 1 ± 0 | <1 | <1 | <1 ± 0 | 9 | 14 | 11.5 ± 3.54 |
| QSAm0.5 | <1 | 1 | 0.5 ± 0.71 | 1 | <1 | 0.5 ± 0.71 | 1 | 2 | 1.5 ± 0.71 |
| QSAm2 | <1 | <1 | 0 ± 0 | <1 | <1 | <1 ± 0 | <1 | 1 | 0.5 ± 0.71 |
| QSAm5 | <1 | <1 | 0 ± 0 | <1 | <1 | <1 ± 0 | <1 | 1 | 0.5 ± 0.71 |
| QSB0.5 | 1 | 1 | 1 ± 0 | 1 | <1 | 0.5 ± 0.71 | <1 | 17 | 8.5 ± 12.02 |
| QSB2 | 2 | <1 | 1 ± 1.41 | <1 | <1 | <1 ± 0 | <1 | 0 | <1 ± 0 |
| QSB5 | 3 | 1 | 2 ± 1.41 | <1 | <1 | <1 ± 0 | 1 | 5 | 3 ± 2.83 |
| QSG0.5 | 2 | 3 | 2.5 ± 0.71 | <1 | <1 | <1 ± 0 | 8 | 3 | 5.5 ± 3.54 |
| QSG2 | <1 | 26 | 13 ± 18.38 | <1 | <1 | <1 ± 0 | 2 | 2 | 2 ± 0 |
| QSG5 | <1 | 31 | 15.5 ± 21.92 | <1 | <1 | <1 ± 0 | 6 | 7 | 6.5 ± 0.71 |
| QSO0.5 | <1 | 1 | 0.5 ± 0.71 | <1 | <1 | <1 ± 0 | 3 | 2 | 2.5 ± 0.71 |
| QSO2 | 10 | <1 | 5 ± 7.07 | <1 | <1 | <1 ± 0 | 3 | 5 | 4 ± 1.41 |
| QSO5 | 3 | 27 | 15 ± 16.97 | <1 | <1 | <1 ± 0 | 4 | 11 | 7.5 ± 4.95 |
| QSP0.5 | <1 | 2 | 1 ± 1.41 | <1 | 2 | 1 ± 1.41 | 2 | <1 | 1 ± 1.41 |
| QSP2 | <1 | 1 | 0.5 ± 0.71 | <1 | <1 | <1 ± 0 | 3 | 1 | 2 ± 1.41 |
| QSP5 | 1 | 3 | 2 ± 1.41 | <1 | <1 | <1 ± 0 | 10 | 1 | 5.5 ± 6.36 |
| QSR0.5 | <1 | <1 | <1 ± 0 | <1 | <1 | <1 ± 0 | <1 | 1 | 0.5 ± 0.71 |
| QSR2 | <1 | <1 | <1 ± 0 | <1 | <1 | <1 ± 0 | 1 | 1 | <1 ± 0 |
| QSR5 | <1 | 1 | 0.5 ± 0.71 | <1 | <1 | <1 ± 0 | 2 | 1 | 1.5 ± 0.71 |
| Code Sample | Aerobic Mesophilic Bacteria | Coliforms | Fungi | |||
|---|---|---|---|---|---|---|
| Approved Test | Inhibition Zone, mm | Approved Test | Inhibition Zone, mm | Approved Test | Inhibition Zone, mm | |
| QS2 | 0/6 | 0 ± 0 | 1/6 | 2 ± 0.89 | 2/6 | 1.333 ± 0.52 |
| QSA0.5 | 5/6 | 2.333 ± 0.52 | 4/6 | 2.333 ± 0.52 | 3/6 | 1.333 ± 0.52 |
| QSA2 | 5/6 | 2.5 ± 0.55 | 4/6 | 2.5 ± 0.55 | 3/6 | 3.5 ± 1.22 |
| QSA5 | 4/6 | 2.5 ± 0.55 | 5/6 | 2.5 ± 0.55 | 5/6 | 8 ± 2.76 |
| QSAm0.5 | 2/6 | 3.667 ± 0.52 | 4/6 | 1.5 ± 0.55 | 1/6 | 6.167 ± 2.40 |
| QSAm2 | 4/6 | 9.333 ± 1.03 | 3/6 | 3.333 ± 0.52 | 4/6 | 6.5 ± 2.35 |
| QSAm5 | 4/6 | 9.5 ± 0.84 | 4/6 | 3.5 ± 0.55 | 4/6 | 7 ± 2.76 |
| QSB0.5 | 4/6 | 1.167 ± 0.41 | 1/6 | 2.667 ± 0.82 | 4/6 | 7 ± 2.76 |
| QSB2 | 4/6 | 1.333 ± 0.52 | 1/6 | 2.833 ± 0.98 | 5/6 | 7.333 ± 2.58 |
| QSB5 | 4/6 | 2.5 ± 0.55 | 1/6 | 3 ± 0.89 | 5/6 | 7.5 ± 2.59 |
| QSG0.5 | 2/6 | 2.333 ± 0.82 | 1/6 | 1.833 ± 0.75 | 2/6 | 7.333 ± 2.58 |
| QSG2 | 5/6 | 4 ± 0.89 | 3/6 | 2.167 ± 0.75 | 0/6 | 7.333 ± 2.58 |
| QSG5 | 6/6 | 4.167 ± 0.98 | 4/6 | 2.167 ± 0.75 | 1/6 | 7.667 ± 2.58 |
| QSO0.5 | 3/6 | 4 ± 0.89 | 2/6 | 1.5 ± 0.55 | 2/6 | 1.5 ± 0.55 |
| QSO2 | 5/6 | 4.167 ± 0.75 | 4/6 | 1.5 ± 0.55 | 3/6 | 1.667 ± 0.52 |
| QSO5 | 6/6 | 4.167 ± 0.98 | 4/6 | 2 ± 0.63 | 4/6 | 1.667 ± 0.52 |
| QSP0.5 | 5/6 | 4 ± 0.89 | 1/6 | 1.833 ± 0.41 | 4/6 | 1.833 ± 0.41 |
| QSP2 | 3/6 | 3.5 ± 0.84 | 1/6 | 1.333 ± 0.52 | 3/6 | 1.333 ± 0.52 |
| QSP5 | 1/6 | 3.333 ± 0.82 | 2/6 | 1.333 ± 0.52 | 0/6 | 1.333 ± 0.52 |
| QSR0.5 | 2/6 | 1.833 ± 0.98 | 5/6 | 6.5 ± 2.35 | 3/6 | 2.5 ± 0.84 |
| QSR2 | 3/6 | 1.833 ± 0.75 | 2/6 | 7.167 ± 2.79 | 3/6 | 6.833 ± 2.93 |
| QSR5 | 4/6 | 2 ± 0.89 | 4/6 | 8 ± 2.76 | 3/6 | 7.167 ± 2.78 |
| Sample | Average Thickness, mm | Sample | Average Thickness, mm |
|---|---|---|---|
| QS2 | 0.164 ± 0.013 | QSG2 | 0.226 ± 0.009 |
| QSA0.5 | 0.194 ± 0.011 | QSG5 | 0.254 ± 0.025 |
| QSA2 | 0.214 ± 0.019 | QSO0.5 | 0.190 ± 0.01 |
| QSA5 | 0.238 ± 0.013 | QSO2 | 0.202 ± 0.013 |
| QSAm0.5 | 0.212 ± 0.015 | QSO5 | 0.194 ± 0.005 |
| QSAm2 | 0.244 ± 0.017 | QSP0.5 | 0.266 ± 0.021 |
| QSAm5 | 0.248 ± 0.043 | QSP2 | 0.216 ± 0.011 |
| QSB0.5 | 0.154 ± 0.005 | QSP5 | 0.272 ± 0.033 |
| QSB2 | 0.162 ± 0.018 | QSR0.5 | 0.184 ± 0.011 |
| QSB5 | 0.226 ± 0.015 | QSR2 | 0.174 ± 0.005 |
| QSG0.5 | 0.236 ± 0.011 | QSR5 | 0.238 ± 0.011 |
| Sample | %T at 280 nm | % T at 400 nm | Transparency | Opacity |
|---|---|---|---|---|
| QS2 | 10.2442 ± 0.71 | 30.8092 ± 2.34 | 9.74382 ± 0.19 | 0.07681 ± 0.01 |
| QSA0.5 | 0.0866 ± 0.070 | 3.94262 ± 1.78 | 7.03310 ± 0.52 | 0.12132 ± 0.0017 |
| QSA2 | 0.9668 ± 0.39 | 11.7966 ± 1.54 | 6.61022 ± 0.19 | 0.11328 ± 0.0084 |
| QSA5 | 0.5295 ± 0.12 | 16.8562 ± 1.90 | 6.50092 ± 0.20 | 0.11983 ± 0.0074 |
| QSAm0.5 | 1.0697 ± 0.33 | 16.5411 ± 1.85 | 6.98136 ± 0.21 | 0.13173 ± 0.02 |
| QSAm2 | 0.2182 ± 0.18 | 8.8739 ± 1.33 | 5.59052 ± 0.09 | 0.20691 ± 0.01 |
| QSAm5 | 0.3032 ± 0.15 | 7.9730 ± 0.82 | 5.39832 ± 0.26 | 0.21030 ± 0.02 |
| QSB0.5 | 2.0982 ± 0.78 | 25.4562 ± 3.24 | 10.3050 ± 0.27 | 0.07983 ± 0.02 |
| QSB2 | 0.2811 ± 0.13 | 11.4734 ± 2.14 | 9.34763 ± 0.24 | 0.11238 ± 0.01 |
| QSB5 | 0.0566 ± 0.05 | 1.1172 ± 0.22 | 4.80696 ± 0.29 | 0.33784 ± 0.02 |
| QSG0.5 | 0.4638 ± 0.15 | 12.8224 ± 0.54 | 6.37701 ± 0.07 | 0.13510 ± 0.01 |
| QSG2 | 0.9107 ± 0.22 | 17.0909 ± 1.57 | 6.8277 ± 0.051 | 0.13645 ± 0.01 |
| QSG5 | 0.1769 ± 0.23 | 1.6192 ± 0.82 | 5.18060 ± 0.67 | 0.25293 ± 0.01 |
| QSO0.5 | 2.0135 ± 0.47 | 17.5357 ± 2.39 | 7.83758 ± 0.25 | 0.08531 ± 0.0039 |
| QSO2 | 0.4690 ± 0.18 | 19.3792 ± 1.36 | 8.50917 ± 0.13 | 0.07273 ± 0.0058 |
| QSO5 | 0.2167 ± 0.29 | 5.6792 ± 1.50 | 8.21114 ± 0.08 | 0.10168 ± 0.0064 |
| QSP0.5 | 0.2437 ± 0.40 | 3.2979 ± 0.40 | 5.9859 ± 0.18 | 0.17956 ± 0.02 |
| QSP2 | 0.0272 ± 0.029 | 0.1323 ± 0.10 | 6.7034 ± 0.52 | 0.20245 ± 0.02 |
| QSP5 | 0.1623 ± 0.10 | 0.1195 ± 0.12 | 4.0516 ± 0.38 | 0.55747 ± 0.07 |
| QSR0.5 | 8.7779 ± 2.05 | 27.2905 ± 3.38 | 8.4157 ± 0.25 | 0.09657 ± 0.0077 |
| QSR2 | 10.9962 ± 4.31 | 26.4296 ± 9.05 | 6.6499 ± 0.81 | 0.09698 ± 0.02 |
| QSR5 | 4.7594 ± 0.22 | 31.5367 ± 3.17 | 7.0720 ± 0.16 | 0.09806 ± 0.01 |
| Compound | pH |
|---|---|
| Acetic acid at 1% (v/v) | 2.60 ± 0.028 |
| Chitosan at 2% (w/v) | 4.46 ± 0 |
| Starch at 2% (w/v) | 5.82 ± 0.014 |
| Cranberry extract | 2.86 ± 0.028 |
| Blueberry extract | 3.29 ± 0.014 |
| Beetroot extract | 4.31 ± 0.014 |
| Pomegranate extract | 3.11 ± 0.014 |
| Oregano extract | 5.24 ± 0 |
| Pitaya extract | 5.14 ± 0.014 |
| Resveratrol extract | 4.93 ± 0.014 |
| Sample | Day 1 | Day 15 |
|---|---|---|
| pH Meter | pH Meter | |
| QS2 | 4.57 ± 0.007 | 5.29 ± 0.021 |
| QSA0.5 | 4.73 ± 0.007 | 5.86 ± 0.014 |
| QSA2 | 4.70 ± 0.007 | 5.54 ± 0.007 |
| QSA5 | 4.68 ± 0.014 | 5.33 ± 0.028 |
| QSAm0.5 | 4.11 ± 0.007 | 4.58 ± 0.014 |
| QSAm2 | 4.07 ± 0.007 | 4.47 ± 0.028 |
| QSAm5 | 4.02 ± 0.007 | 4.34 ± 0.007 |
| QSB0.5 | 4.83 ± 0.007 | 5.88 ± 0.007 |
| QSB2 | 4.78 ± 0.007 | 5.60 ± 0.028 |
| QSB5 | 4.62 ± 0.014 | 5.59 ± 0.007 |
| QSG0.5 | 4.52 ± 0.021 | 6.14 ± 0.014 |
| QSG2 | 4.41 ± 0 | 6.02 ± 0.035 |
| QSG5 | 4.31 ± 0.014 | 5.97 ± 0.007 |
| QSO0.5 | 4.81 ± 0.007 | 5.89 ± 0.014 |
| QSO2 | 4.77 ± 0.014 | 5.58 ± 0.035 |
| QSO5 | 4.62 ± 0.014 | 5.52 ± 0.007 |
| QSP0.5 | 4.01 ± 0.007 | 5.26 ± 0.007 |
| QSP2 | 4.04 ± 0.014 | 5.46 ± 0.014 |
| QSP5 | 4.09 ± 0.014 | 4.83 ± 0.014 |
| QSR0.5 | 4.65 ± 0.007 | 5.92 ± 0.021 |
| QSR2 | 4.62 ± 0.007 | 5.88 ± 0.007 |
| QSR5 | 4.59 ± 0.007 | 5.77 ± 0.028 |
| Factor | p Value | Statistical Implication | Conclusion |
|---|---|---|---|
| AOX | <0.0005 | Reject null hypothesis of no difference in means. | At least one mean pH is significantly different from the rest because of the type of antioxidant that was incorporated into the film. |
| % AOX | 0.002 | Reject null hypothesis of no difference in means. | At least one mean pH is significantly different from the rest because of the content of antioxidant (% wt) in the film. |
| Day | <0.0005 | Reject null hypothesis of no difference in means. | The mean pH measured on day 1 is significantly different from that measured on day 15. |
| AOX and % AOX | 0.991 | Do not reject null hypothesis of no interaction between the factors. | The effect of the type of antioxidant on the mean pH does not significantly depend on its content (% wt). |
| AOX and day | <0.0005 | Reject null hypothesis of no interaction between the factors. | The effect of the type of antioxidant on the mean pH significantly depends on the day when the pH is measured. |
| % AOX and day | 0.175 | Do not reject null hypothesis of no interaction between the factors. | The effect of the content of antioxidant (% wt) on the mean pH does not significantly depend on the day when the pH is measured. |
| AOX, % AOX and day | <0.0005 | Reject null hypothesis of no interaction among the factors. | There is a significant effect of the combination of the three factors in the mean pH values. |
| Sample | Storage Modulus (E’) | Tg °C | Tan δ | |
|---|---|---|---|---|
| Average Value (MPa) | Average Value | |||
| 50 °C | 100 °C | |||
| QS2 | 4753 | 3093 | 185.2 | 0.5405 |
| QSA0.5 | 3001 | 1967 | 158.4 | 0.330 |
| QSA2 | 3868 | 2633 | 90.1 | 0.166 |
| QSA5 | 2982 | 2065 | 87.1 | 0.151 |
| QSAm5 | 2214 | 497.17 | 104 | 0.361 |
| QSG5 | 2407 | 665 | 102 | 0.331 |
| QSO5 | 494 | 250 | 93.3 | 0.459 |
| QSP0.5 | 1668 | 838 | 102.1 | 0.321 |
| QSP5 | 925 | 376 | 95.3 | 0.364 |
| QSR5 | 441 | 179 | 88.6 | 0.542 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, Optical and Mechanical Properties of Chitosan–Starch Films with Natural Extracts. Int. J. Mol. Sci. 2017, 18, 997. https://doi.org/10.3390/ijms18050997
Lozano-Navarro JI, Díaz-Zavala NP, Velasco-Santos C, Martínez-Hernández AL, Tijerina-Ramos BI, García-Hernández M, Rivera-Armenta JL, Páramo-García U, Reyes-de la Torre AI. Antimicrobial, Optical and Mechanical Properties of Chitosan–Starch Films with Natural Extracts. International Journal of Molecular Sciences. 2017; 18(5):997. https://doi.org/10.3390/ijms18050997
Chicago/Turabian StyleLozano-Navarro, Jessica I., Nancy P. Díaz-Zavala, Carlos Velasco-Santos, Ana L. Martínez-Hernández, Beatriz I. Tijerina-Ramos, Margarita García-Hernández, José L. Rivera-Armenta, Ulises Páramo-García, and Adriana I. Reyes-de la Torre. 2017. "Antimicrobial, Optical and Mechanical Properties of Chitosan–Starch Films with Natural Extracts" International Journal of Molecular Sciences 18, no. 5: 997. https://doi.org/10.3390/ijms18050997