Neuroendocrine Differentiation in Metastatic Conventional Prostate Cancer Is Significantly Increased in Lymph Node Metastases Compared to the Primary Tumors
Abstract
:1. Introduction
2. Results
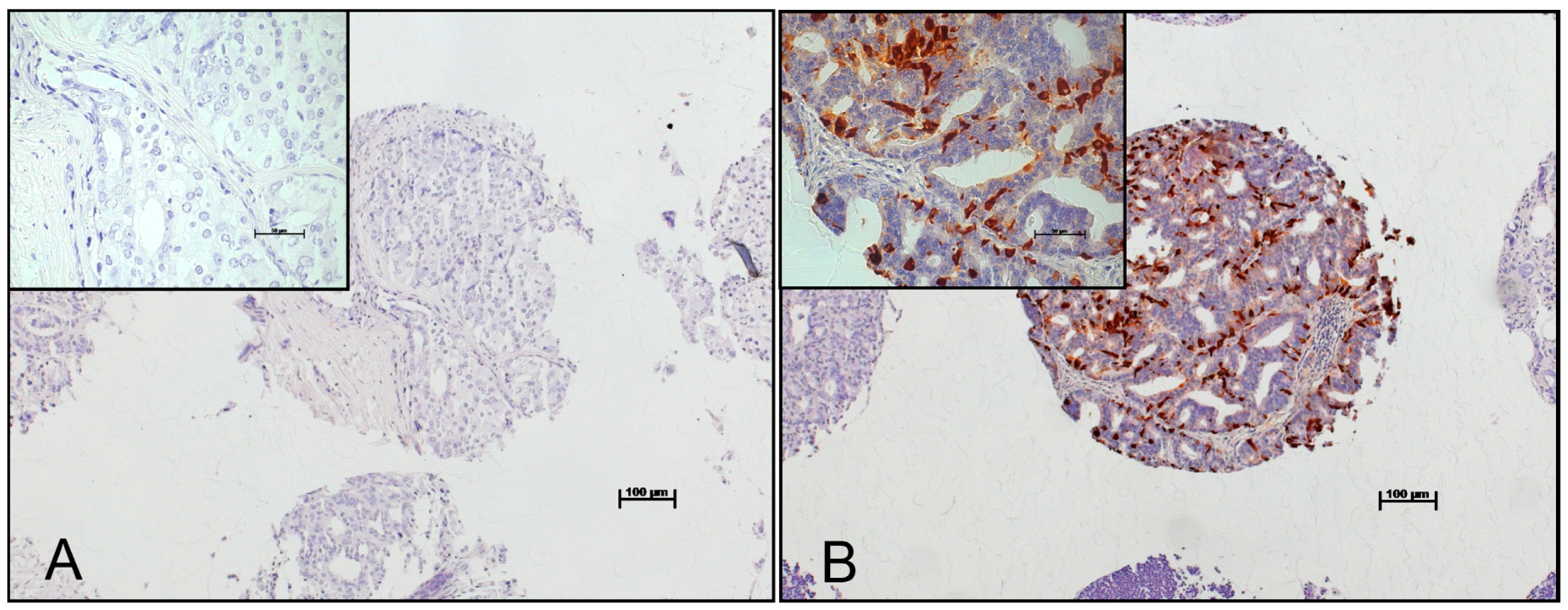
2.1. Patient Characteristics and Expression of Chromogranin A in Benign Prostate, Primary Tumors and Lymph Node Metastases Considering the Gleason Patterns
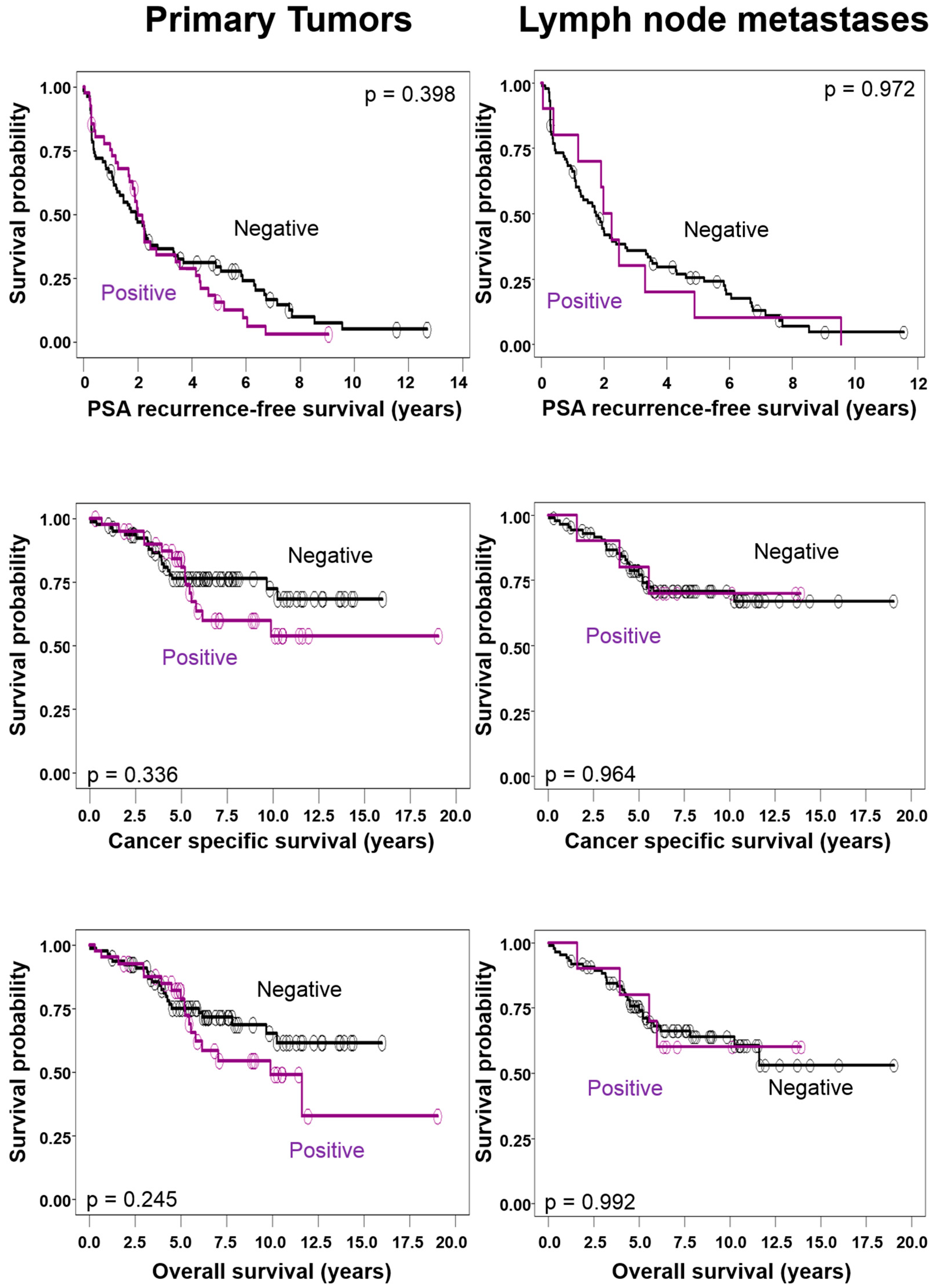
2.2. Correlations of Chromogranin A Expression in Primary Tumors and Lymph Node Metastases with Clinico-Pathological Tumor Characteristics and Survival
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Surgical Technique of Lymphadenectomy
4.3. Pathology
4.4. Tissue Microarray
4.5. Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NED | Neuroendocrine Differentiation |
| NE | Neuroendocrine |
| GP | Gleason Pattern |
| CgA | Chromogranin A |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| TMA | Tissue Microarray |
| PSA | Prostate-Specific Antigen |
References
- Epstein, J.I.; Amin, M.B.; Evans, A.J.; Huang, J.; Rubin, M.A. Neuroendocrine tumours. In WHO Classification of Tumours of the Urinary System and Male Genital Organs; Moch, H., Humphrey, P.A., Ulbright, T.M., Reuter, V.E., Eds.; IARC: Lyon, France, 2016. [Google Scholar]
- Bonkhoff, H.; Stein, U.; Remberger, K. Multidirectional differentiation in the normal, hyperplastic, and neoplastic human prostate: Simultaneous demonstration of cell-specific epithelial markers. Hum. Pathol. 1994, 25, 42–46. [Google Scholar] [CrossRef]
- Yuan, T.C.; Veeramani, S.; Lin, M.F. Neuroendocrine-like prostate cancer cells: Neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr. Relat. Cancer 2007, 14, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Priemer, D.S.; Montironi, R.; Wang, L.; Williamson, S.R.; Lopez-Beltran, A.; Cheng, L. Neuroendocrine tumors of the prostate: Emerging insights from molecular data and updates to the 2016 world health organization classification. Endocr. Pathol. 2016, 27, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, A.; Cardi, A.; Dattilo, C.; Mariotti, G.; di Monaco, F.; di Silverio, F. New perspective in the management of neuroendocrine differentiation in prostate adenocarcinoma. Int. J. Clin. Pract. 2006, 60, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Komiya, A.; Suzuki, H.; Imamoto, T.; Kamiya, N.; Nihei, N.; Naya, Y.; Ichikawa, T.; Fuse, H. Neuroendocrine differentiation in the progression of prostate cancer. Int. J. Urol. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.D.; Ben-Jacob, E.; Farach-Carson, M.C. Prostate cancer and neuroendocrine differentiation: More neuronal, less endocrine? Front. Oncol. 2015, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Yao, Y.H.; Li, B.G.; Tang, Y.; Chang, J.W.; Zhang, J. Neuroendocrine prostate cancer (NEPC) progressing from conventional prostatic adenocarcinoma: Factors associated with time to development of NEPC and survival from NEPC diagnosis—A systematic review and pooled analysis. J. Clin. Oncol. 2014, 32, 3383–3390. [Google Scholar] [PubMed]
- Epstein, J.I.; Amin, M.B.; Beltran, H.; Lotan, T.L.; Mosquera, J.-M.; Reuter, V.E.; Robinson, B.D.; Troncoso, P.; Rubin, M.A. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am. J. Surg. Pathol. 2014, 38, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Vignani, F.; Russo, L.; Bertaglia, V.; Tullio, M.; Tucci, M.; Poggio, M.; Dogliotti, L. Prognostic role of neuroendocrine differentiation in prostate cancer, putting together the pieces of the puzzle. Open Access J. Urol. 2010, 2, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Appetecchia, M.; Meçule, A.; Pasimeni, G.; Iannucci, C.V.; de Carli, P.; Baldelli, R.; Barnabei, A.; Cigliana, G.; Sperduti, I.; Gallucci, M. Incidence of high Chromogranin A serum levels in patients with non metastatic prostate adenocarcinoma. J. Exp. Clin. Cancer Res. 2010, 29, 166. [Google Scholar] [CrossRef] [PubMed]
- Burgio, S.L.; Conteduca, V.; Menna, C.; Carretta, E.; Rossi, L.; Bianchi, E.; Kopf, B.; Fabbri, F.; Amadori, D.; de Giorgi, U. Chromogranin A predicts outcome in prostate cancer patients treated with abiraterone. Endocr. Relat. Cancer 2014, 21, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Burgio, S.L.; Menna, C.; Carretta, E.; Rossi, L.; Bianchi, E.; Masini, C.; Amadori, D.; de Giorgi, U. Chromogranin A is a potential prognostic marker in prostate cancer patients treated with enzalutamide. Prostate 2014, 74, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, A.; di Silverio, F.; Autran, A.M.; Salciccia, S.; Gentilucci, A.; Alfarone, A.; Gentile, V. Distribution of high Chromogranin A serum levels in patients with nonmetastatic and metastatic prostate adenocarcinoma. Urol. Int. 2009, 82, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Aprikian, A.G.; Cordon-Cardo, C.; Fair, W.R.; Reuter, V.E. Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer 1993, 71, 3952–3965. [Google Scholar] [CrossRef]
- Aprikian, A.G.; Cordon-Cardo, C.; Fair, W.R.; Zhang, Z.F.; Bazinet, M.; Hamdy, S.M.; Reuter, V.E. Neuroendocrine differentiation in metastatic prostatic adenocarcinoma. J. Urol. 1994, 151, 914–919. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Qian, J.; Pacelli, A.; Zincke, H.; Blute, M.; Bergstralh, E.J.; Slezak, J.M.; Cheng, L. Neuroendocrine expression in node positive prostate cancer: Correlation with systemic progression and patient survival. J. Urol. 2002, 168, 1204–1211. [Google Scholar] [CrossRef]
- Cheville, J.C.; Tindall, D.; Boelter, C.; Jenkins, R.; Lohse, C.M.; Pankratz, V.S.; Sebo, T.J.; Davis, B.; Blute, M.L. Metastatic prostate carcinoma to bone. Cancer 2002, 95, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Quek, M.L.; Daneshmand, S.; Rodrigo, S.; Cai, J.; Dorff, T.B.; Groshen, S.; Skinner, D.G.; Lieskovsky, G.; Pinski, J. Prognostic significance of neuroendocrine expression in lymph node-positive prostate cancer. Urology 2006, 67, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Roudier, M.P.; True, L.D.; Higano, C.S.; Vesselle, H.; Ellis, W.; Lange, P.; Vessella, R.L. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum. Pathol. 2003, 34, 646–653. [Google Scholar] [CrossRef]
- McWilliam, L.J.; Manson, C.; George, N.J. Neuroendocrine differentiation and prognosis in prostatic adenocarcinoma. Br. J. Urol. 1997, 80, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Pruneri, G.; Galli, S.; Rossi, R.S.; Roncalli, M.; Coggi, G.; Ferrari, A.; Simonato, A.; Siccardi, A.G.; Carboni, N.; Buffa, R. Chromogranin A and B and secretogranin II in prostatic adenocarcinomas: Neuroendocrine expression in patients untreated and treated with androgen deprivation therapy. Prostate 1998, 34, 113–120. [Google Scholar] [CrossRef]
- Angelsen, A.; Syversen, U.; Haugen, O.A.; Stridsberg, M.; Mjølnerød, O.K.; Waldum, H.L. Neuroendocrine differentiation in carcinomas of the prostate: Do neuroendocrine serum markers reflect immunohistochemical findings? Prostate 1997, 30, 1–6. [Google Scholar] [CrossRef]
- Fleischmann, A.; Schobinger, S.; Schumacher, M.; Thalmann, G.N.; Studer, U.E. Survival in surgically treated, nodal positive prostate cancer patients is predicted by histopathological characteristics of the primary tumor and its lymph node metastases. Prostate 2009, 69, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Brawn, P.N.; Speights, V.O. The dedifferentiation of metastatic prostate carcinoma. Br. J. Cancer 1989, 59, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Slezak, J.; Bergstralh, E.J.; Cheville, J.C.; Sweat, S.; Zincke, H.; Bostwick, D.G. Dedifferentiation in the metastatic progression of prostate carcinoma. Cancer 1999, 86, 657–663. [Google Scholar] [CrossRef]
- Abrahamsson, P.A. Neuroendocrine differentiation in prostatic carcinoma. Prostate 1999, 39, 135–148. [Google Scholar] [CrossRef]
- Mucci, N.R.; Akdas, G.; Manely, S.; Rubin, M.A. Neuroendocrine expression in metastatic prostate cancer: Evaluation of high throughput tissue microarrays to detect heterogeneous protein expression. Hum. Pathol. 2000, 31, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Bader, P.; Burkhard, F.C.; Markwalder, R.; Studer, U.E. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J. Urol. 2003, 169, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, A.; Schobinger, S.; Markwalder, R.; Schumacher, M.; Burkhard, F.; Thalmann, G.N.; Studer, U.E. Prognostic factors in lymph node metastases of prostatic cancer patients: The size of the metastases but not extranodal extension independently predicts survival. Histopathology 2008, 53, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Helpap, B.; Algaba, F.; Humphrey, P.A.; Allsbrook, W.C.; Iczkowski, K.A.; Bastacky, S.; Lopez-Beltran, A.; Boccon-Gibod, L.; Montironi, R.; et al. Acinar adenocarcinoma. In Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; Eble, J.N., Sauter, G., Epstein, J.I., Sesterhenn, I.A., Eds.; IARC Press: Lyon, France, 2004. [Google Scholar]
- Green, F.L. TNM Classification of Malignant Tumors; Bierley, J., Gospodarowicz, M.K., Wittekind, C., Eds.; Wiley-Blackwell: Oxford, UK, 2017. [Google Scholar]
- Søreide, K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J. Clin. Pathol. 2008, 62, 1–5. [Google Scholar] [CrossRef] [PubMed]

| Patient Data (n = 119) | |
| Age (median, range) at surgery (years) | 65 (45–75) |
| Follow-up (median, range) (years) | 5.9 (0.1–15.2) |
| Patients with biochemical failure at last follow-up (n) | 103 |
| Patients dead of disease at last follow-up (n) | 33 |
| Patients dead at last follow-up (n) | 40 |
| Prostatectomy Data | |
| pT2 (n) | 14 |
| pT3a (n) | 55 |
| pT3b (n) | 50 |
| Prostate cancer volume (median, range) (cm3) | 12.6 (0.66–127) |
| Gleason score 6 (n) | 12 |
| Gleason score 7 (n) | 63 |
| Gleason score 8 (n) | 21 |
| Gleason score 9 (n) | 23 |
| Lymphadenectomy Data | |
| Evaluated nodes per patient (median, range) (n) | 22 (9–68) |
| Positive nodes per patient (median, range) (n) | 2 (1–24) |
| CgA Expression | Parameters of the Primary Tumor (Mean ± SD) | Parameters of Nodal Metastases (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Age | p | Tumor volume (cm3) | p | Total size (mm) | p | Total number | p | |
| Primary Tumor | ||||||||
| CgA negative | 64.4 ± 6.1 | 0.978 | 18.0 ± 15.4 | 0.821 | 19.6 ± 34.8 | 0.989 | 3.3 ± 3.8 | 0.813 |
| CgA positive | 64.3 ± 5.8 | 21.5 ± 24.9 | 17.2 ± 24.4 | 3.0 ± 3.3 | ||||
| Nodal Metastases | ||||||||
| CgA negative | 64.3 ± 5.9 | 0.027 | 19.1 ± 19.5 | 0.819 | 19.4 ± 31.7 | 0.458 | 3.3 ± 3.4 | 0.279 |
| CgA positive | 59.3 ± 6.3 | 18.9 ± 13.7 | 36.4 ± 49.4 | 5.3 ± 6.9 | ||||
| Parameter | Cut-Off | Overall Survival | Disease-Specific Survival | Recurrence-Free Survival | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| CgA in Primary Tumor | Positive | 1.0 | 0.132 | 1.0 | 0.241 | 1.0 | 0.66 |
| Negative | 1.65 (0.9–3.1) | 1.54 (0.8–3.1) | 1.1 (0.7–1.7) | ||||
| Metastases size | <7.5 mm | 1.0 | <0.001 | 1.0 | 0.002 | 1.0 | 0.036 |
| ≥7.5 mm | 4.34 (2.0–9.6) | 4.12 (1.7–10.0) | 1.58 (1.1–2.4) | ||||
| Gleason score | 6 to 8 | 1.0 | 0.571 | 1.0 | 0.375 | 1.0 | 0.074 |
| 9 to 10 | 1.23 (0.6–2.5) | 1.41 (0.7–3.0) | 1.57 (1.0–2.6) | ||||
| CgA in Nodal Metastases | Positive | 1.0 | 0.571 | 1.0 | 0.5 | 1.0 | 0.327 |
| Negative | 0.73 (0.2–2.2) | 0.65 (0.2–2.8) | 0.69 (0.3–1.4) | ||||
| Metastases size | <7.5 mm | 1.0 | <0.001 | 1.0 | 0.003 | 1.0 | 0.063 |
| ≥7.5 mm | 5.3 (2.0–14.1) | 6.44 (1.9–22.1) | 1.58 (1.0–2.5) | ||||
| Gleason score | 6 to 8 | 1.0 | 0.365 | 1.0 | 1.88 | 1.0 | 0.082 |
| 9 to 10 | 1.43 (0.7–3.1) | 1.75 (0.8–4.0) | 1.62 (0.9–2.8) | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genitsch, V.; Zlobec, I.; Seiler, R.; Thalmann, G.N.; Fleischmann, A. Neuroendocrine Differentiation in Metastatic Conventional Prostate Cancer Is Significantly Increased in Lymph Node Metastases Compared to the Primary Tumors. Int. J. Mol. Sci. 2017, 18, 1640. https://doi.org/10.3390/ijms18081640
Genitsch V, Zlobec I, Seiler R, Thalmann GN, Fleischmann A. Neuroendocrine Differentiation in Metastatic Conventional Prostate Cancer Is Significantly Increased in Lymph Node Metastases Compared to the Primary Tumors. International Journal of Molecular Sciences. 2017; 18(8):1640. https://doi.org/10.3390/ijms18081640
Chicago/Turabian StyleGenitsch, Vera, Inti Zlobec, Roland Seiler, George N. Thalmann, and Achim Fleischmann. 2017. "Neuroendocrine Differentiation in Metastatic Conventional Prostate Cancer Is Significantly Increased in Lymph Node Metastases Compared to the Primary Tumors" International Journal of Molecular Sciences 18, no. 8: 1640. https://doi.org/10.3390/ijms18081640




