Zinc Differentially Modulates the Assembly of Soluble and Polymerized Vimentin
Abstract
:1. Introduction
2. Results
2.1. EDTA-Free Vimentin Reveals Differential Polymerization Features Derived from the Presence of the Cysteine Residue
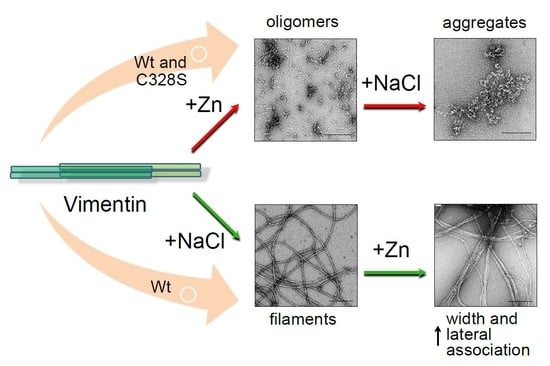
2.2. Micromolar Zinc Reversibly induces Vimentin Oligomerization
2.3. The Morphology of Zinc-Induced Vimentin Assemblies Depends on the Support
2.4. Assessment of Zinc-Induced Vimentin Structures by Electron Microscopy
2.5. Effect of Zinc on NaCl-Induced Polymerization and on Preformed Filaments
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EM | Electron microscopy |
| TPEN | N, N, N’, N’ tetrakis (2-pyridylmethyl) ethylenediamine |
| ULF | Unit length filament |
| wt | Wild-type |
References
- Gan, Z.; Ding, L.; Burckhardt, C.J.; Lowery, J.; Zaritsky, A.; Sitterley, K.; Mota, A.; Costigliola, N.; Starker, C.G.; Voytas, D.F.; et al. Vimentin Intermediate Filaments Template Microtubule Networks to Enhance Persistence in Cell Polarity and Directed Migration. Cell Syst. 2016, 3, 252–263.e8. [Google Scholar] [CrossRef] [Green Version]
- Duarte, S.; Viedma-Poyatos, Á.; Navarro-Carrasco, E.; Martínez, A.E.; Pajares, M.A.; Pérez-Sala, D. Vimentin filaments interact with the actin cortex in mitosis allowing normal cell division. Nat. Commun. 2019, 10, 4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sala, D.; Oeste, C.L.; Martínez, A.E.; Jesús Carrasco, M.; Garzón, B.; Javier Cañada, F. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat. Commun. 2015, 6, 7287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mónico, A.; Duarte, S.; Pajares, M.A.; Pérez-Sala, D. Vimentin disruption by lipoxidation and electrophiles: Role of the cysteine residue and filament dynamics. Redox Biol. 2019, 23, 101098. [Google Scholar] [CrossRef] [PubMed]
- Antfolk, D.; Sjöqvist, M.; Cheng, F.; Isoniemi, K.; Duran, C.L.; Rivero-Muller, A.; Antila, C.; Niemi, R.; Landor, S.; Bouten, C.V.C.; et al. Selective regulation of Notch ligands during angiogenesis is mediated by vimentin. Proc. Natl. Acad. Sci. USA 2017, 114, E4574–E4581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patteson, A.E.; Vahabikashi, A.; Pogoda, K.; Adam, S.A.; Mandal, K.; Kittisopikul, M.; Sivagurunathan, S.; Goldman, A.; Goldman, R.D.; Janmey, P.A. Vimentin protects cells against nuclear rupture and DNA damage during migration. J. Cell Biol. 2019, 218, 4079–4092. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Hao, Y.; Zheng, T.; Gupta, S.K.; Parada, G.A.; Wu, H.; Lin, S.; Wang, S.; Zhao, X.; et al. High stretchability, strength, and toughness of living cells enabled by hyperelastic vimentin intermediate filaments. Proc. Natl. Acad. Sci. USA 2019, 116, 17175–17180. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, M.; Tanaka, H.; Inoko, A.; Goto, H.; Yonemura, S.; Kobori, K.; Hayashi, Y.; Kondo, E.; Itohara, S.; Izawa, I.; et al. Defect of mitotic vimentin phosphorylation causes microophthalmia and cataract via aneuploidy and senescence in lens epithelial cells. J. Biol. Chem. 2013, 288, 35626–35635. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [Green Version]
- Mak, T.N.; Bruggemann, H. Vimentin in Bacterial Infections. Cells 2016, 5, 18. [Google Scholar] [CrossRef]
- Musaelyan, A.; Lapin, S.; Nazarov, V.; Tkachenko, O.; Gilburd, B.; Mazing, A.; Mikhailova, L.; Shoenfeld, Y. Vimentin as antigenic target in autoimmunity: A comprehensive review. Autoimmun. Rev. 2018, 17, 926–934. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Structure and Assembly. Cold Spring Harbor Perspect. Biol. 2016, 8, a018242. [Google Scholar] [CrossRef]
- Premchandar, A.; Mücke, N.; Poznański, J.; Wedig, T.; Kaus-Drobek, M.; Herrmann, H.; Dadlez, M. Structural Dynamics of the Vimentin Coiled-coil Contact Regions Involved in Filament Assembly as Revealed by Hydrogen-Deuterium Exchange. J. Biol. Chem. 2016, 291, 24931–24950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, C.G.; Saldanha, O.; Aufderhorst-Roberts, A.; Martinez-Torres, C.; Kuijs, M.; Koenderink, G.H.; Köster, S.; Huber, K. Effect of ionic strength on the structure and elongational kinetics of vimentin filaments. Soft Matter 2018, 14, 8445–8454. [Google Scholar] [CrossRef] [PubMed]
- Snider, N.T.; Omary, M.B. Post-translational modifications of intermediate filament proteins: Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 163–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, K.R.; Herrmann, H.; Franke, W.W. Characterization of disulfide crosslink formation of human vimentin at the dimer, tetramer, and intermediate filament levels. J. Struct. Biol. 1996, 117, 55–69. [Google Scholar] [CrossRef]
- Janmey, P.A.; Slochower, D.R.; Wang, Y.H.; Wen, Q.; Cēbers, A. Polyelectrolyte properties of filamentous biopolymers and their consequences in biological fluids. Soft Matter 2014, 10, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Broedersz, C.P.; Rowat, A.C.; Wedig, T.; Herrmann, H.; Mackintosh, F.C.; Weitz, D.A. Divalent cations crosslink vimentin intermediate filament tail domains to regulate network mechanics. J. Mol. Biol. 2010, 399, 637–644. [Google Scholar] [CrossRef]
- Brennich, M.E.; Bauch, S.; Vainio, U.; Wedig, T.; Herrmann, H.; Köster, S. Impact of ion valency on the assembly of vimentin studied by quantitative small angle X-ray scattering. Soft Matter 2014, 10, 2059–2068. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Shen, Y.; Wang, D. Effect of the divalent cations zinc and calcium on the structure and mechanics of reconstituted vimentin intermediate filaments. BioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, I.; Herrmann, H.; Franke, W.W. Assembly and structure of calcium-induced thick vimentin filaments. Eur. J. Cell Biol. 1991, 56, 328–341. [Google Scholar] [PubMed]
- Kooijman, M.; Bloemendal, M.; Traub, P.; van Grondelle, R.; van Amerongen, H. Transient electric birefringence study of intermediate filament formation from vimentin and glial fibrillary acidic protein. J. Biol. Chem. 1997, 272, 22548–22555. [Google Scholar] [CrossRef] [Green Version]
- Brennich, M.E.; Vainio, U.; Wedig, T.; Bauch, S.; Herrmann, H.; Köster, S. Mutation-induced alterations of intra-filament subunit organization in vimentin filaments revealed by SAXS. Soft Matter 2019, 15, 1999–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dammann, C.; Noding, B.; Koster, S. Vimentin networks at tunable ion-concentration in microfluidic drops. Biomicrofluidics 2012, 6, 22009–2200910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dammann, C.; Koster, S. Dynamics of counterion-induced attraction between vimentin filaments followed in microfluidic drops. Lab Chip 2014, 14, 2681–2687. [Google Scholar] [CrossRef] [Green Version]
- Kwak, H.I.; Kang, H.; Dave, J.M.; Mendoza, E.A.; Su, S.C.; Maxwell, S.A.; Bayless, K.J. Calpain-mediated vimentin cleavage occurs upstream of MT1-MMP membrane translocation to facilitate endothelial sprout initiation. Angiogenesis 2012, 15, 287–303. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, N.; Goto, H.; Ogawara, M.; Nishi, Y.; Ando, S.; Inagaki, M. Spatial patterns of Ca2+ signals define intracellular distribution of a signaling by Ca2+/Calmodulin-dependent protein kinase II. J. Biol. Chem. 1997, 272, 25195–25199. [Google Scholar] [CrossRef] [Green Version]
- Heimfarth, L.; da Silva Ferreira, F.; Pierozan, P.; Loureiro, S.O.; Mingori, M.R.; Moreira, J.C.F.; da Rocha, J.B.T.; Pessoa-Pureur, R. Calcium signaling mechanisms disrupt the cytoskeleton of primary astrocytes and neurons exposed to diphenylditelluride. Biochim. Biophys. Acta 2016, 1860, 2510–2520. [Google Scholar] [CrossRef]
- Maret, W. New perspectives of zinc coordination environments in proteins. J. Inorg. Biochem. 2012, 111, 110–116. [Google Scholar] [CrossRef]
- Stefano, L.S.; Pierre, P.; Jae-Young, K.; Elias, A.; Ashley, I. Bush and Michal Hershfinkel. The neurophysiology and pathology of brain zinc. J. Neurosci. 2011, 31, 16076–16085. [Google Scholar]
- Mo, Z.Y.; Zhu, Y.Z.; Zhu, H.L.; Fan, J.B.; Chen, J.; Liang, Y. Low micromolar zinc accelerates the fibrillization of human tau via bridging of Cys-291 and Cys-322. J. Biol. Chem. 2009, 284, 34648–34657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell. Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellomo, E.; Abro, A.; Hogstrand, C.; Maret, W.; Domene, C. Role of Zinc and Magnesium Ions in the Modulation of Phosphoryl Transfer in Protein Tyrosine Phosphatase 1B. J. Am. Chem. Soc. 2018, 140, 4446–4454. [Google Scholar] [CrossRef] [Green Version]
- Maret, W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid Redox Signal. 2006, 8, 1419–1441. [Google Scholar] [CrossRef]
- Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, K.; Murozuka, T.; Caldwell, R.; Epstein, W.L. Divalent cation stimulation of in vitro fibre assembly from epidermal keratin protein. J. Cell Sci. 1978, 33, 255–263. [Google Scholar]
- Mack, J.W.; Steven, A.C.; Steinert, P.M. The mechanism of interaction of filaggrin with intermediate filaments. The ionic zipper hypothesis. J. Mol. Biol. 1993, 232, 50–66. [Google Scholar] [CrossRef]
- Pérez-Sala, D.; Oeste, C.L.; Sánchez-Gómez, F.J. Vimentin gets a new glow from zinc. Oncotarget 2015, 6, 15742–15743. [Google Scholar]
- Mónico, A.; Zorrilla, S.; Pérez-Sala, D. Characterization of vimentin-zinc interaction and its impact on the response to electrophilic and oxidative stress. Free Rad. Biol. Med. 2017, 108, S46. [Google Scholar] [CrossRef]
- Mónico, A.; Martínez-Senra, E.; Cañada, F.J.; Zorrilla, S.; Pérez-Sala, D. Drawbacks of dialysis procedures for removal of EDTA. PLoS ONE 2017, 12, e0169843. [Google Scholar] [CrossRef] [PubMed]
- Mücke, N.; Wedig, T.; Bürer, A.; Marekov, L.N.; Steinert, P.M.; Langowski, J.; Aebi, U.; Herrmann, H. Molecular and biophysical characterization of assembly-starter units of human vimentin. J. Mol. Biol. 2004, 340, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch Biochem Biophys 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolova, A.V.; Kreplak, L.; Wedig, T.; Mücke, N.; Svergun, D.I.; Herrmann, H.; Aebi, U.; Strelkov, S.V. Monitoring intermediate filament assembly by small-angle x-ray scattering reveals the molecular architecture of assembly intermediates. Proc. Natl. Acad. Sci. USA 2006, 103, 16206–16211. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, J.C.; London, E. Inadvertent concentrating of EDTA by ion exchange chromatography: Avoiding artifacts that can interfere with protein purification. Anal. Biochem. 1997, 250, 124–125. [Google Scholar] [CrossRef]
- Pace, N.J.; Weerapana, E. A competitive chemical-proteomic platform to identify zinc-binding cysteines. ACS Chem. Biol. 2014, 9, 258–265. [Google Scholar] [CrossRef]
- Lengyel, I.; Flinn, J.M.; Peto, T.; Linkous, D.H.; Cano, K.; Bird, A.C.; Lanzirotti, A.; Frederickson, C.J.; van Kuijk, F.J. High concentration of zinc in sub-retinal pigment epithelial deposits. Exp. Eye Res. 2007, 84, 772–780. [Google Scholar] [CrossRef]
- Flinn, J.M.; Kakalec, P.; Tappero, R.; Jones, B.; Lengyel, I. Correlations in distribution and concentration of calcium, copper and iron with zinc in isolated extracellular deposits associated with age-related macular degeneration. Met. Integr. Biometal Sci. 2014, 6, 1223–1228. [Google Scholar] [CrossRef]
- Thompson, R.B.; Reffatto, V.; Bundy, J.G.; Kortvely, E.; Flinn, J.M.; Lanzirotti, A.; Jones, E.A.; McPhail, D.S.; Fearn, S.; Boldt, K.; et al. Identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proc. Natl. Acad. Sci. USA 2015, 112, 1565–1570. [Google Scholar] [CrossRef] [Green Version]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.; Bergen, A.A. The dynamic nature of Bruch’s membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.Y.; Zhang, D.L.; Liu, X.L.; Li, X.S.; Cheng, X.Q.; Chen, J.; Du, H.N.; Liang, Y. Pathological concentration of zinc dramatically accelerates abnormal aggregation of full-length human Tau and thereby significantly increases Tau toxicity in neuronal cells. Biochim. Biophys Acta Mol. Basis Dis. 2017, 1863, 414–427. [Google Scholar] [CrossRef] [PubMed]
- McCord, M.C.; Aizenman, E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, R.; Farabella, I.; Schumacher, F.F.; Miller, A.; Gor, J.; Martin, A.C.; Jones, D.T.; Lengyel, I.; Perkins, S.J. Zinc binding to the Tyr402 and His402 allotypes of complement factor H: Possible implications for age-related macular degeneration. J. Mol. Biol. 2011, 408, 714–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eagle, G.R.; Zombola, R.R.; Himes, R.H. Tubulin-zinc interactions: Binding and polymerization studies. Biochemistry 1983, 22, 221–228. [Google Scholar] [CrossRef]
- Melki, R.; Carlier, M.F. Thermodynamics of tubulin polymerization into zinc sheets: Assembly is not regulated by GTP hydrolysis. Biochemistry 1993, 32, 3405–3413. [Google Scholar] [CrossRef]
- Mackenzie, G.G.; Salvador, G.A.; Romero, C.; Keen, C.L.; Oteiza, P.I. A deficit in zinc availability can cause alterations in tubulin thiol redox status in cultured neurons and in the developing fetal rat brain. Free Radic. Biol. Med. 2011, 51, 480–489. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, H.; Hofmann, I.; Franke, W.W. Identification of a nonapeptide motif in the vimentin head domain involved in intermediate filament assembly. J. Mol. Biol. 1992, 223, 637–650. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mónico, A.; Zorrilla, S.; Rivas, G.; Pérez-Sala, D. Zinc Differentially Modulates the Assembly of Soluble and Polymerized Vimentin. Int. J. Mol. Sci. 2020, 21, 2426. https://doi.org/10.3390/ijms21072426
Mónico A, Zorrilla S, Rivas G, Pérez-Sala D. Zinc Differentially Modulates the Assembly of Soluble and Polymerized Vimentin. International Journal of Molecular Sciences. 2020; 21(7):2426. https://doi.org/10.3390/ijms21072426
Chicago/Turabian StyleMónico, Andreia, Silvia Zorrilla, Germán Rivas, and Dolores Pérez-Sala. 2020. "Zinc Differentially Modulates the Assembly of Soluble and Polymerized Vimentin" International Journal of Molecular Sciences 21, no. 7: 2426. https://doi.org/10.3390/ijms21072426