Preparation and Characterization of Highly Fluorescent, Glutathione-coated Near Infrared Quantum Dots for in Vivo Fluorescence Imaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fluorescence and absorption spectra of hydrophobic CdSeTe/CdS QDs
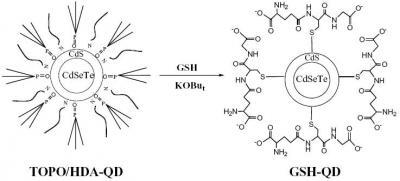
2.2. Surface coating of hydrophobic CdSeTe/CdS QDs with glutathione (GSH)
2.3. Fluorescence brightness of GSH-coated CdSeTe/CdS QD and its stability in PBS buffer
2.4. Hydrodynamic diameter and dispersibility of GSH-coated CdSeTe/CdS QDs in PBS buffer
2.5. Cellular uptake and cell viability of GSH-coated CdSeTe/CdS QDs
2.6. In vivo lymph node imaging using GSH-coated CdSeTe/CdS QDs
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Synthesis of CdSeTe/CdS QDs
4.3. Preparation of GSH-, MUA- and PEI-coated CdSeTe/CdS QDs
4.4. Characterization of QDs
4.5. Cellular uptake and viability test
4.6. In vivo lymph node imaging
Acknowledgments
References and Notes
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar]
- Liu, H.; Beauvoit, B; Kimura, M; Chance, B. Dependence of tissue optical properties on solute-induced changes in refractive index and osmolarity. J. Biomed. Opt. 1996, 1, 200–211. [Google Scholar]
- Lim, YT; Kim, S; Nakayama, A; Stott, NE; Bawendi, MG; Frangioni, JV. Selection of quantum dot wavelengths for biomedical assays and imaging. Mol. Imaging 2003, 2, 50–64. [Google Scholar]
- Gómez-Hens, A; Aguilar-Caballos, MP. Long-wavelength fluorophores: new trends in their analytical use. Trends Anal. Chem 2004, 23, 127–136. [Google Scholar]
- Amiot, CL; Xu, S; Liang, S; Pan, L; Zhao, JX. Near-infrared fluorescent materials for sensing of biological targets. Sensors 2008, 8, 3082–3105. [Google Scholar]
- Benson, RC; Kues, HA. Fluorescence properties of indocyanine green as related to angiography. Phys. Med. Biol. 1978, 23, 159–163. [Google Scholar]
- Saxena, V; Sadoqi, M; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar]
- Cai, W; Chen, X. Preparation of peptide-conjugated quantum dots for tumor vasculature-targeted imaging. Nat. Protocol 2008, 3, 89–96. [Google Scholar]
- Rao, J; Dragulescu-Andrasi, A; Yao, H. Fluorescence imaging in vivo: recent advances. Curr. Opin. Biotechnol 2007, 18, 17–25. [Google Scholar]
- Iga, AM; Robertson, JH; Winslet, MC; Seifalian, AM. Clinical potential of quantum dots. J. Biomed. Biotechnol 2007, 1–10. [Google Scholar]
- Gao, X; Yang, L; Petros, JA; Marshall, FF; Simons, JW; Nie, S. In vivo molecular and celluar imaging with quantum dots. Curr. Opin. Biotechnol 2005, 16, 63–72. [Google Scholar]
- Michalet, X; Pinaud, FF; Bentolila, LA; Tsay, JM; Doose, S; Li, JJ; Sundaresan, G; Wu, AM; Gambhir, SS; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar]
- Medintz, IL; Uyeda, HT; Goldman, ER; Mattoussi, H. Quantum dot bioconjugates for imaging, labeling and sensing. Nat. Mater 2005, 4, 435–446. [Google Scholar]
- Jiang, W; Papa, E; Fisher, H; Mardyani, S; Chan, WCW. Semiconductor quantum dots as contrast agents for whole animal imaging. Trends Biotechnol 2004, 22, 607–609. [Google Scholar]
- Smith, AM; Gao, X; Nie, S. Quantum dot nanocrystals for in vivo molecular and cellular imaging. Photochem. Photobio 2004, 80, 377–385. [Google Scholar]
- Frangioni, JV. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol 2003, 7, 626–634. [Google Scholar]
- Kim, S; Lim, YT; Soltesz, EG; Grand, AMD; Lee, J; Nakayama, A; Parker, JA; Mihaljevic, T; Laurence, RG; Dor, DM; Cohn, LH; Bawendi, MG; Frangioni, JV. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004, 22, 93–97. [Google Scholar]
- Morgan, NY; English, S; Chen, W; Chernomordik, V; Russo, A; Smith, PD; Gandjbakhche, A. Real time in vivo non-invasive optical imaging using near-infrared fluorescent quantum dots. Acad. Radiol 2005, 12, 313–323. [Google Scholar]
- Hinds, S; Myrskog, S; Levina, L; Koleilat, G; Yang, J; Kelley, SO; Sargent, EH. NIR-emitting colloidal quantum dots having 26% luminescence quantum yield in buffer solution. J. Am. Chem. Soc 2007, 129, 7218–7219. [Google Scholar]
- Hyun, B-R; Chen, H; Rey, DA; Wise, FW; Batt, CA. Near-infrared fluorescence imaging with water-soluble lead salt quantum dots. J. Phys. Chem. B 2007, 111, 5726–5730. [Google Scholar]
- Acharya, S; Gautam, UK; Sasaki, T; Bando, Y; Golan, Y; Ariga, K. Ultra narrow PbS nanorods with intense fluorescence. J. Am. Chem. Soc 2008, 130, 4594–4595. [Google Scholar]
- Bailey, RE; Nie, S. Alloyed semiconductor quantum dots: tuning the optical properties without changing the particle size. J. Am. Chem. Soc 2003, 12, 7100–7106. [Google Scholar]
- Bailey, RE; Strausburg, JB; Nie, S. A new class of far-red and near-infrared biological labels based on alloyed semiconductor quantum dots. J. Nanosci. Nanotech 2004, 4, 569–574. [Google Scholar]
- Jiang, W; Singhal, A; Zheng, J; Wang, C; Chan, WCW. Optimizing the synthesis of red- to near-ir-emitting CdS-capped CdTexSe1-x alloyed quantum dots for biomedical imaging. Chem. Mater 2006, 18, 4845–4854. [Google Scholar]
- Tsay, JM; Pflughoefft, M; Bentolila, LA; Weiss, S. Hybrid approach to the synthesis of highly luminescent CdTe/ZnS and CdHgTe/ZnS nanocrystals. J. Am. Chem. Soc 2004, 126, 1926–1927. [Google Scholar]
- Kim, S-W; Zimmer, JP; Ohnishi, S; Tracy, JB; Frangioni, JV; Bawendi, MG. Engineering InAsxP1-x/InP/ZnSe III-V alloyed core/shell quantum dots for the near-infrared. J. Am. Chem. Soc 2005, 127, 10526–10532. [Google Scholar]
- Murray, CB; Norris, DJ; Bawendi, MG. Synthesis and characterization of nearly monodisperse CdE (E = S, Se, Te) semiconductor nanocrystallites. J. Am. Chem. Soc 1993, 115, 8706–8715. [Google Scholar]
- Dabbousi, BO; Rodriguez-Viejo, J; Mikulec, FV; Heine, JR; Mattoussi, H; Ober, R; Jensen, KF; Bawendi, MG. (CdSe)ZnS core-shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar]
- Pathak, S; Choi, S-K; Arnheim, N; Thompson, ME. Hydroxylated quantum dots as luminescent probes for in situ hybridization. J. Am. Chem. Soc 2001, 123, 4103–4104. [Google Scholar]
- Kim, S; Bawendi, MG. Oligomeric ligands for luminescent and stable nanocrystal quantum dots. J. Am. Chem. Soc 2003, 125, 14652–14653. [Google Scholar]
- Jin, T; Fujii, F; Sakata, H; Tamura, M; Kinjo, M. Calixarene-coated water-soluble CdSe-ZnS semiconductor quantum dots that are highly fluorescent and stable in aqueous solution. Chem. Commun 2005, 2829–2831. [Google Scholar]
- Kirchner, C; Liedl, T; Kudera, S; Pellegrino, T; Javier, AM; Gaub, HE; Stölzle, S; Fertig, N; Parak, WJ. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett 2005, 5, 331–338. [Google Scholar]
- Kim, S; Fisher, B; Eisler, H-J; Bawendi, M. Type II quantum dots: CdTe/CdSe(core/shell) and CdSe/ZnTe(core/shell) heterostructures. J. Am. Chem. Soc 2003, 125, 11466–11467. [Google Scholar]
- Qian, H; Dong, C; Peng, J; Qiu, X; Xu, Y; Ren, J. Highly-quality and water-soluble near-infrared photoluminescent CdHgTe/CdS quantum dots prepared by adjusting size and composition. J. Phys. Chem. C 2007, 111, 16852–16857. [Google Scholar]
- Zheng, Y; Gao, S; Ying, JY. Synthesis and cell-imaging applications of glutathione-capped CdTe Quantum dots. Adv. Mater 2007, 19, 376–380. [Google Scholar]
- Zheng, Y; Yang, Z; Ying, JY. Aqueous synthesis of glutathione-capped ZnSe and Zn1-xCdxSe alloyed quantum dots. Adv. Mater 2007, 19, 1475–1479. [Google Scholar]
- Jiang, C; Xu, S; Yang, D; Zhang, F; Wang, W. Synthesis of glutathione-capped CdS quantum dots and preliminary studies on protein detection and cell fluorescence image. Luminescence 2007, 22, 430–437. [Google Scholar]
- Gill, R; Bahshi, L; Freeman, R; Willner, I. Optical detection of glucose and acetylcholine esterase inhibitors by H2O2-sensitive CdSe/ZnS quantum dots. Angew. Chem. Int. Ed. 2008, 47, 1676–1679. [Google Scholar]
- Singhal, RK; Anderson, ME; Meister, A. Glutathione, a first line of defense against cadmium toxicity. FASEB J 1987, 1, 220–223. [Google Scholar]
- Hermanson, GT. Bioconjugate Techniques; Academic press: New York, 1996; pp. 137–226. [Google Scholar]
- Aldana, J; Wang, YA; Peng, X. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J. Am. Chem. Soc 2001, 123, 8844–8850. [Google Scholar]
- Thelwall, PE; Yemin, AY; Gillian, TL; Simpson, NE; Kasibhatla, MS; Rabbani, ZN; Macdonald, JM; Blackband, SJ; Gamcsik, MP. Nonivasive in vivo detection of glutathione metabolism in tumors. Cancer Res 2005, 65, 10149–10153. [Google Scholar]
- Satoh, T; Yshioka, Y. Contribution of reduced and oxidized glutathione to signals detected by magnetic resonance spectroscopy as indicators of local brain redox state. Neurosci. Res 2006, 55, 34–39. [Google Scholar]
- DeNardo, SJ; Yao, Z; Lam, KS; Song, A; Burke, PA; Mirick, GR; Lamborn, KR; O’Donnell, RT; DeNardo, GL. Effect of molecular size of pegylated peptide on the pharmacokinetics and tumor targeting in lymphoma-bearing mice. Clin. Can. Res. 2003, 9, 3854s–3864s. [Google Scholar]
- Liu, W; Choi, HS; Zimmer, JP; Tanaka, E; Frangioni, JV; Bawendi, M. Compact cysteine-coated CdSe(ZnCdS) quantum dots for in vivo applications. J. Am. Chem. Soc 2007, 129, 14530–14531. [Google Scholar]
- Liu, W; Howarth, M; Greytak, AB; Zheng, Y; Nocera, DG; Ting, AY; Bawendi, MG. Compact biocompatible quantum dots functionalized for cellular imaging. J. Am. Chem. Soc 2008, 130, 1274–1284. [Google Scholar]
- Howarth, M; Liu, W; Puthenveetil, S; Zheng, Y; Marshall, LF; Schmidt, MM; Wittrup, KD; Bawendi, MG. Monovalent, reduced-size quantum dots for imaging receptors on living cells. Nat. Methods 2008, 5, 397–399. [Google Scholar]
- Kobayashi, H; Hama, Y; Koyama, Y; Barrett, T; Regino, CAS; Urano, Y; Choyke, PL. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano Lett 2007, 7, 1711–1716. [Google Scholar]
- Jin, T; Fujii, F; Yamada, E; Nodasaka, Y; Kinjo, M. Control of the optical properties of quantum dots by surface coating with calix[n]arene carboxylic acids. J. Am. Chem. Soc 2006, 128, 9288–9289. [Google Scholar]
- Heuff, RF; Swift, JL; Cramb, DT. Fluorescence correlation spectroscopy using quantum dots: advances, challenges and opportunities. Phys. Chem. Chem. Phys 2007, 9, 1870–1880. [Google Scholar]
- Qu, L; Peng, ZA; Peng, X. Alternative routes toward high quality CdSe nanocrystals. Nano Lett 2001, 1, 333–337. [Google Scholar]
- Qu, L; Peng, X. Control of photoluminescence properties of CdSe nanocrystals in growth. J. Am. Chem. Soc. 2002, 124, 2049–2055. [Google Scholar]










© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jin, T.; Fujii, F.; Komai, Y.; Seki, J.; Seiyama, A.; Yoshioka, Y. Preparation and Characterization of Highly Fluorescent, Glutathione-coated Near Infrared Quantum Dots for in Vivo Fluorescence Imaging. Int. J. Mol. Sci. 2008, 9, 2044-2061. https://doi.org/10.3390/ijms9102044
Jin T, Fujii F, Komai Y, Seki J, Seiyama A, Yoshioka Y. Preparation and Characterization of Highly Fluorescent, Glutathione-coated Near Infrared Quantum Dots for in Vivo Fluorescence Imaging. International Journal of Molecular Sciences. 2008; 9(10):2044-2061. https://doi.org/10.3390/ijms9102044
Chicago/Turabian StyleJin, Takashi, Fumihiko Fujii, Yutaka Komai, Junji Seki, Akitoshi Seiyama, and Yoshichika Yoshioka. 2008. "Preparation and Characterization of Highly Fluorescent, Glutathione-coated Near Infrared Quantum Dots for in Vivo Fluorescence Imaging" International Journal of Molecular Sciences 9, no. 10: 2044-2061. https://doi.org/10.3390/ijms9102044