A New Approach on Estimation of Solubility and n-Octanol/ Water Partition Coefficient for Organohalogen Compounds
Abstract
:1. Introduction
2. Methodology
3. Regression Analysis
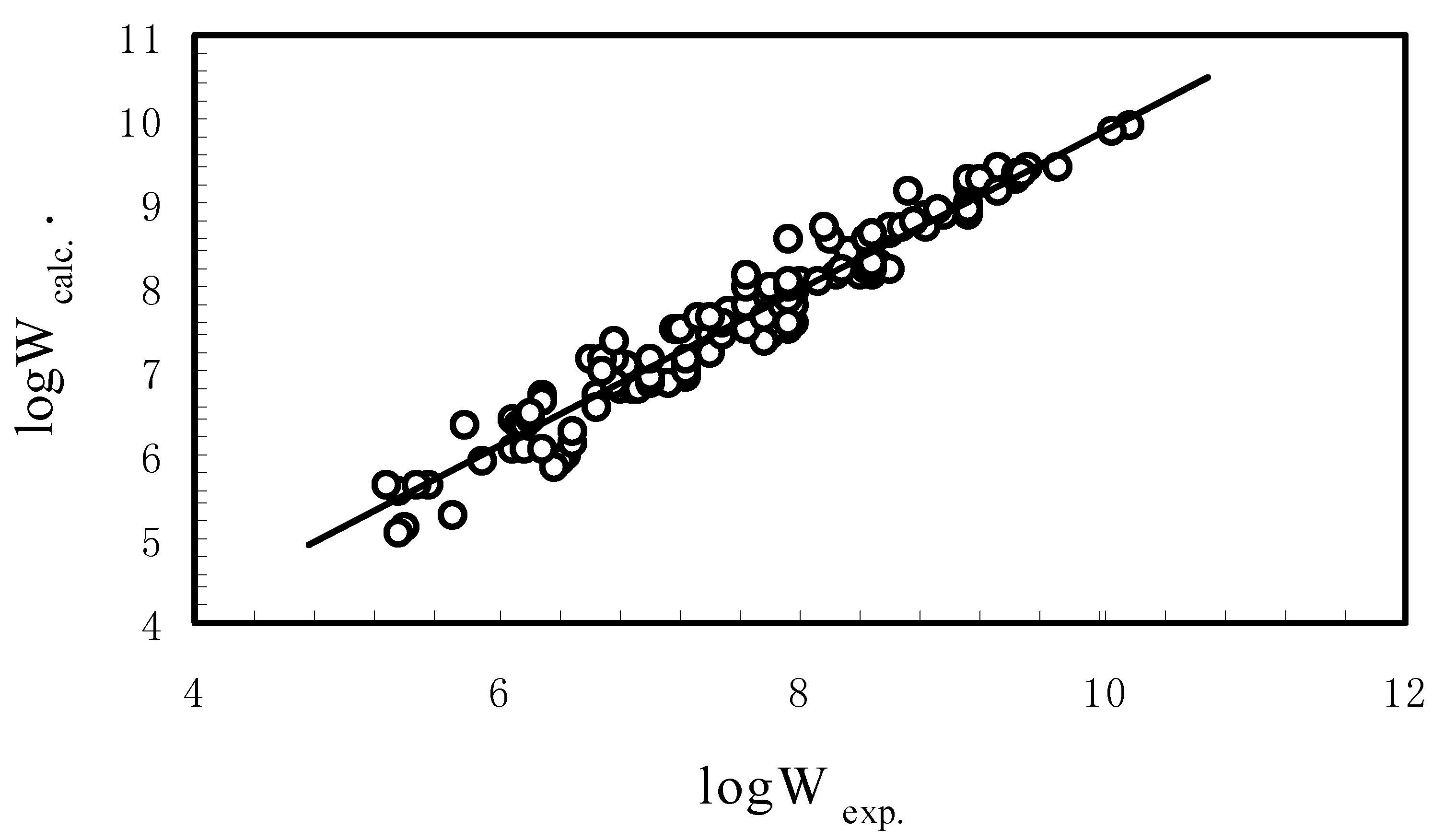
3.1. Aqueous Solubility of PCBs
3.2. n-Octanol/Water Partition Coefficient of PCBs
4. Discussion
5. Conclusions
Acknowledgment
References and Notes
- Hantsch, C. Quantitative approach to biochemical structure-activity relationships. Acc. Chem. Res. 1969, 2, 232–239. [Google Scholar]
- Bodor, N; Huang, MJ. A new method for the estimation of the aqueous solubility of organic compounds. J. Pharm. Sci. 1992, 81, 954–960. [Google Scholar]
- Hansch, C; Leo, A. Substituent Constant for Correlation in Chemical and Biology; Wiley-Interscience: New York, NY, USA, 1979. [Google Scholar]
- Cao, C; Li, ZL. Molecular Polarizability. 1. Relationship to Water Solubility of Alkanes and Alcohols. J. Chem. Inf. Comput. Sci. 1998, 38, 1–7. [Google Scholar]
- Bodor, N; Gabanyi, Z; Wong, CK. A new method for the estimation of partition coefficient. J. Am. Chem. Soc. 1989, 111, 3783–3786. [Google Scholar]
- Jain, N; Yalkowsky, SH. UPPER III: Unified physical property estimation relationships. Application to non-hydrogen bonding aromatic compounds. J. Pharm. Sci. 1999, 88, 852–860. [Google Scholar]
- Huuskonen, JJ; Villa, AEP; Tetko, IV. Prediction of partition coefficient based on atom-type electrotopological state indices. J. Pharm. Sci. 1999, 88, 229–233. [Google Scholar]
- Hall, LH; Kier, LB. Electrotopological State Indices for Atom Types: A Novel Combination of Electronic, Topological, and Valence State Information. J. Chem. Inf. Comput. Sci. 1995, 35, 1039–1045. [Google Scholar]
- Abraham, MH. Scales of solute hydrogen-bonding: their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 1993, 22, 73–83. [Google Scholar]
- Ruelle, P. The n-octanol and n-hexane/water partition coefficient of environmentally relevant chemicals predicted from the mobile order and disorder (MOD) thermodynamics. Chemosphere 2000, 40, 457–512. [Google Scholar]
- Wang, X; Tang, S; Liu, S; et al. Molecular Hologram Derived Quantitative Structure–Property Relationships to Predict Physico-Chemical Properties of Polychlorinated Biphenyls. Chemosphere 2003, 51, 617–632. [Google Scholar]
- Staikova, M; Wania, F; Donaldson, DJ. Molecular polarizability as a single-parameter predictor of vapour pressures and octanol–air partitioning coefficients of non-polar compounds: a priori approach and results. Atmos. Environ. 2004, 38, 213–225. [Google Scholar]
- Gramatica, P; Navas, N; Todeschini, R. 3D-modelling and prediction by WHIM descriptors. Part 9. Chromatographic relative retention time and physico-chemical properties of polychlorinated biphenyls (PCBs). Chemometr. Intell. Lab. 1998, 40, 53–63. [Google Scholar]
- Hansen, BG; Paya-Perez, AB; Rahman, M; Larsen, BR. QSARs for KOW and KOC of PCB congeners: a critical examination of data, assumptions and statistical approaches. Chemosphere 1999, 39, 2209–2228. [Google Scholar]
- Tolls, J; van Dijk, J; Verbruggen, EJM; et al. Aqueous Solubility-Molecular Size Relationships: A Mechanistic Case Study Using C10- to C19-Alkanes. J. Phys. Chem. A. 2002, 106, 2760–2765. [Google Scholar]
- Kamlet, MJ; Doherty, RM; Abraham, MH; et al. Linear solvation energy relationship. 46. An improved equation for correlation and prediction of octanol/water partition coefficients of organic nonelectrolytes (including strong hydrogen bond donor solutes). J. Phys. Chem. 1988, 92, 5244–5255. [Google Scholar]
- Huang, J; Yu, G; Zhang, ZL; et al. Application of TLSER method in predicting the aqueous solubility and n - octanol/water partition coefficient of PCBs, PCDDs and PCDFs. J. Environ. Sci. 2004, 16, 21–29. [Google Scholar]
- Lin, ZH; Liu, SS; Li, ZL; et al. QSPR Study on Solubility and Octanol/Water Partition Coefficients of Hydrocarbons by Molecular Distance-Edge Vector. Environ. Chem. 2000, 19, 528–537. (in Chinese). [Google Scholar]
- Dai, JY; Chen, L; Zhao, JF. Prediction of partition property of polychlorinated organic compounds using linear solvation energy parameter. China Environ. Sci. 2002, 22, 329–333. (in Chinese). [Google Scholar]
- Jorgensen, WL; Duffy, EM. Prediction of drug solubility from structure. Adv. Drug Deliver. Rev. 2002, 54, 355–366. [Google Scholar]
- Kühen, R; Ebert, RU; Schüürmann, G. Model Selection Based on Structural Similarity-Method Description and Application to Water Solubility Prediction. J. Chem. Inf. Model. 2006, 46, 636–641. [Google Scholar]
- Ballschmiter, KH; Haltrich, W; Kühn, W; et al. HOV-Studie: Halogenorganische Verbindungen in Gewässern; Fachgruppe Wasserchemie der Gesellschaft Deutscher Chemiker: Frankfurt, 1987. [Google Scholar]
- Padmanabhan, J; Parthasarathi, R; Subramanian, V; Chattaraj, PK. QSPR models for polychlorinated biphenyls: n-Octanol/water partition coefficient. Bioorg. Med. Chem. 2006, 14, 1021–1028. [Google Scholar]
- Lü, WJ; Chen, YL; Liu, MC; et al. QSPR prediction of n-octanol/water partition coefficient for polychlorinated biphenyls. Chemosphere 2007, 69, 469–478. [Google Scholar]
- Zuo, JW; Jiang, YJ; Hu, GX; et al. QSPR/QSAR Studies on the Physicochemical Properties and Biological Activities of Polychlorinated Biphenyls. Acta Phys.-Chim. Sin. 2005, 21, 267–272. (in Chinese). [Google Scholar]
- Kamlet, MJ; Doherty, RM; Abboud, JLM; et al. Solubility–a new look. Chemtech 1986, 16, 566–576. [Google Scholar]
- Hansch, C; Quinlan, JE; Lawrence, GL. Linear free-energy relationship between partition coefficients and the aqueous solubility of organic liquids. J. Org. Chem. 1968, 33, 347–350. [Google Scholar]
- Chou, JT; Jurs, PC. Computer-Assisted Computation of Partition Coefficients from Molecular Structures Using Fragment Constants. J. Chem. Inf. Comput. Sci. 1979, 19, 172–178. [Google Scholar]

| Substitution patterns | V | EHOMO (a.u.) | ELUMO (a.u.) | -logWexp.a | logPOW exp.b | -logWcalc.c | logPOW calc.d | -ΔlogWe | ΔlogPOWe |
|---|---|---|---|---|---|---|---|---|---|
| Chlorobiphenyls | |||||||||
| 2- | 172.9 | −0.31684 | 0.11773 | 4.38 | 4.82 | 4.55 | −0.17 | ||
| 3- | 172.9 | −0.31044 | 0.10490 | 5.39 | 4.66 | 5.15 | 4.71 | 0.24 | −0.05 |
| 4- | 172.9 | −0.30610 | 0.10507 | 5.33 | 4.63 | 5.08 | 4.67 | 0.25 | −0.04 |
| 2,2’- | 185.8 | −0.33398 | 0.12177 | 5.72 | 4.72 | 5.31 | 4.89 | 0.41 | −0.17 |
| 2,3- | 185.8 | −0.32596 | 0.10991 | 5.35 | 4.99 | 5.58 | 5.02 | −0.23 | −0.03 |
| 2,3’- | 185.8 | −0.32760 | 0.10802 | 5.26 | 4.84 | 5.66 | 5.07 | −0.40 | −0.23 |
| 2,4- | 185.8 | −0.32248 | 0.10594 | 5.56 | 5.15 | 5.66 | 5.06 | −0.10 | 0.09 |
| 2,4’- | 185.8 | −0.32189 | 0.10697 | 5.46 | 5.09 | 5.62 | 5.04 | −0.16 | 0.05 |
| 2,5- | 185.8 | −0.32464 | 0.10552 | 5.00 | 5.70 | 5.09 | −0.09 | ||
| 2,6- | 185.8 | −0.33322 | 0.11642 | 4.93 | 5.47 | 4.97 | −0.04 | ||
| 3,3’- | 185.8 | −0.32076 | 0.09420 | 6.45 | 5.27 | 6.02 | 5.25 | 0.43 | 0.02 |
| 3,4- | 185.8 | −0.31429 | 0.09528 | 6.39 | 5.23 | 5.89 | 5.17 | 0.50 | 0.06 |
| 3,4’- | 185.8 | −0.31601 | 0.09419 | 6.40 | 5.15 | 5.95 | 5.21 | 0.45 | −0.06 |
| 3,5- | 185.8 | −0.32035 | 0.09364 | 5.40 | 6.03 | 5.25 | 0.15 | ||
| 4,4’- | 185.8 | −0.31190 | 0.09439 | 6.37 | 5.23 | 5.88 | 5.17 | 0.49 | 0.06 |
| 2,2’,3- | 198.7 | −0.33898 | 0.11209 | 6.10 | 5.12 | 6.07 | 5.36 | 0.03 | −0.24 |
| 2,2’,4- | 198.7 | −0.34012 | 0.11041 | 6.49 | 5.39 | 6.14 | 5.40 | 0.35 | −0.01 |
| 2,2’,5- | 198.7 | −0.33646 | 0.11028 | 6.17 | 5.33 | 6.09 | 5.37 | 0.08 | −0.04 |
| 2,2’,6- | 198.7 | −0.33558 | 0.11438 | 5.90 | 5.04 | 5.94 | 5.29 | −0.04 | −0.25 |
| 2,3,3’- | 198.7 | −0.33701 | 0.10200 | 5.60 | 6.37 | 5.52 | 0.08 | ||
| 2,3,4- | 198.7 | −0.32989 | 0.09988 | 6.18 | 5.68 | 6.33 | 5.49 | −0.15 | 0.19 |
| 2,3,4’- | 198.7 | −0.33020 | 0.10032 | 5.80 | 5.29 | 6.32 | 5.49 | −0.52 | −0.20 |
| 2,3,6- | 198.7 | −0.33795 | 0.10464 | 6.49 | 5.44 | 6.29 | 5.48 | 0.20 | −0.04 |
| 2,3’,4- | 198.7 | −0.33268 | 0.09730 | 6.11 | 5.54 | 6.46 | 5.56 | −0.35 | −0.02 |
| 2,3’,5- | 198.7 | −0.33037 | 0.09875 | 6.14 | 5.65 | 6.38 | 5.52 | −0.24 | 0.13 |
| 2,4,4’- | 198.7 | −0.33669 | 0.09729 | 6.22 | 5.71 | 6.52 | 5.59 | −0.30 | 0.12 |
| 2,4,5- | 198.7 | −0.33641 | 0.10953 | 5.74 | 6.11 | 5.38 | 0.36 | ||
| 2,4,6- | 198.7 | −0.32716 | 0.09635 | 5.50 | 6.41 | 5.53 | −0.03 | ||
| 2,4’,5- | 198.7 | −0.32866 | 0.09554 | 6.18 | 5.68 | 6.46 | 5.56 | −0.28 | 0.12 |
| 2,4’,6- | 198.7 | −0.33949 | 0.10293 | 6.16 | 5.24 | 6.37 | 5.52 | −0.21 | −0.28 |
| 2,3’,4’- | 198.7 | −0.32913 | 0.09591 | 6.21 | 5.71 | 6.45 | 5.55 | −0.24 | 0.16 |
| 2,3’,5’- | 198.7 | −0.33555 | 0.10933 | 6.30 | 5.71 | 6.11 | 5.38 | 0.19 | 0.33 |
| 3,3’,5- | 198.7 | −0.33039 | 0.08369 | 5.70 | 6.87 | 5.77 | −0.07 | ||
| 3,4,4’- | 198.7 | −0.31950 | 0.08533 | 5.22 | 6.66 | 5.65 | −0.43 | ||
| 2,2’,3,3’- | 211.6 | −0.34469 | 0.10442 | 6.83 | 5.67 | 6.77 | 5.80 | 0.06 | −0.13 |
| 2,2’,3,4- | 211.6 | −0.34347 | 0.10180 | 7.00 | 5.79 | 6.84 | 5.84 | 0.16 | −0.05 |
| 2,2’,3,4’- | 211.6 | −0.34602 | 0.10288 | 6.96 | 5.72 | 6.84 | 5.84 | 0.12 | −0.12 |
| 2,2’,3,5’- | 211.6 | −0.34073 | 0.10278 | 6.91 | 5.73 | 6.76 | 5.80 | 0.15 | −0.07 |
| 2,2’,3,6- | 211.6 | −0.33988 | 0.10317 | 6.30 | 4.84 | 6.74 | 5.78 | −0.44 | −0.94 |
| 2,2’,3,6’- | 211.6 | −0.34164 | 0.10791 | 6.30 | 4.84 | 6.61 | 5.72 | −0.31 | −0.88 |
| 2,2’,4,4’- | 211.6 | −0.34756 | 0.10135 | 7.23 | 5.94 | 6.91 | 5.88 | 0.32 | 0.06 |
| 2,2’,4,5- | 211.6 | −0.34328 | 0.10017 | 6.86 | 5.69 | 6.89 | 5.86 | −0.03 | −0.17 |
| 2,2’,4,5’- | 211.6 | −0.34181 | 0.10125 | 7.12 | 5.87 | 6.83 | 5.83 | 0.29 | 0.04 |
| 2,2’,4,6- | 211.6 | −0.34116 | 0.10154 | 6.94 | 5.75 | 6.81 | 5.82 | 0.13 | −0.07 |
| 2,2’,4,6’- | 211.6 | −0.34355 | 0.10650 | 6.65 | 5.51 | 6.68 | 5.76 | −0.03 | −0.25 |
| 2,2’,5,5’- | 211.6 | −0.34136 | 0.10118 | 7.00 | 5.79 | 6.82 | 5.83 | 0.18 | −0.04 |
| 2,2’,5,6’- | 211.6 | −0.33758 | 0.10649 | 6.65 | 5.55 | 6.60 | 5.71 | 0.05 | −0.16 |
| 2,2’,6,6’- | 211.6 | −0.34062 | 0.11159 | 6.20 | 5.24 | 6.47 | 5.65 | −0.27 | −0.41 |
| 2,3,3’,4- | 211.6 | −0.34043 | 0.09287 | 6.77 | 6.10 | 7.08 | 5.97 | −0.31 | 0.13 |
| 2,3,4,4’- | 211.6 | −0.33393 | 0.09127 | 6.86 | 6.24 | 7.04 | 5.94 | −0.18 | 0.30 |
| 2,3,4,5- | 211.6 | −0.33570 | 0.08962 | 6.05 | 7.12 | 5.98 | 0.07 | ||
| 2,3,4’,5- | 211.6 | −0.33694 | 0.08918 | 6.77 | 6.10 | 7.15 | 6.00 | −0.38 | 0.10 |
| 2,3,4’,6- | 211.6 | −0.33980 | 0.09821 | 7.02 | 5.76 | 6.90 | 5.87 | 0.12 | −0.11 |
| 2,3,5,6- | 211.6 | −0.34219 | 0.09374 | 7.25 | 5.96 | 7.08 | 5.97 | 0.17 | −0.01 |
| 2,3’,4,4’- | 211.6 | −0.33518 | 0.08905 | 6.63 | 5.98 | 7.13 | 5.99 | −0.50 | −0.01 |
| 2,3’,4,5- | 211.6 | −0.33790 | 0.08747 | 6.32 | 7.22 | 6.04 | 0.28 | ||
| 2,3’,4,6- | 211.6 | −0.34162 | 0.09677 | 7.26 | 6.03 | 6.97 | 5.91 | 0.29 | 0.12 |
| 2,3’,4’,5- | 211.6 | −0.33665 | 0.08847 | 6.69 | 6.22 | 7.17 | 6.01 | −0.48 | 0.21 |
| 2,3’,4’,6- | 211.6 | −0.33738 | 0.08940 | 7.02 | 5.76 | 7.15 | 6.00 | −0.13 | −0.24 |
| 2,4,4’,5- | 211.6 | −0.34486 | 0.10227 | 6.77 | 6.10 | 6.84 | 5.84 | −0.07 | 0.26 |
| 2,4,4’,6- | 211.6 | −0.33283 | 0.08676 | 7.26 | 6.03 | 7.17 | 6.01 | 0.09 | 0.02 |
| 2,3’,4’,5’- | 211.6 | −0.34118 | 0.09656 | 6.71 | 5.98 | 6.97 | 5.91 | −0.26 | 0.07 |
| 3,3’,4,4’- | 211.6 | −0.32696 | 0.07703 | 6.21 | 7.40 | 6.12 | 0.09 | ||
| 3,3’,5,5’- | 211.6 | −0.34046 | 0.07418 | 6.10 | 7.70 | 6.28 | −0.18 | ||
| 2,2’,3,3’,6- | 224.5 | −0.34538 | 0.09793 | 6.78 | 5.60 | 7.36 | 6.19 | −0.58 | −0.59 |
| 2,2’,3,4,4’- | 224.5 | −0.35109 | 0.09425 | 7.62 | 6.18 | 7.56 | 6.30 | 0.06 | −0.12 |
| 2,2’,3,4,5- | 224.5 | −0.34736 | 0.09147 | 7.87 | 6.38 | 7.60 | 6.31 | 0.27 | 0.07 |
| 2,2’,3,4,5’- | 224.5 | −0.34484 | 0.09419 | 7.66 | 6.23 | 7.47 | 6.25 | 0.19 | −0.02 |
| 2,2’,3,4,6- | 224.5 | −0.34424 | 0.09240 | 7.92 | 6.50 | 7.52 | 6.27 | 0.40 | 0.23 |
| 2,2’,3,4,6’- | 224.5 | −0.34923 | 0.09995 | 6.78 | 5.60 | 7.35 | 6.18 | −0.57 | −0.58 |
| 2,2’,3,4’,5- | 224.5 | −0.34906 | 0.09275 | 7.82 | 6.32 | 7.58 | 6.31 | 0.24 | 0.01 |
| 2,2’,3,4’,6- | 224.5 | −0.34780 | 0.09405 | 7.17 | 5.87 | 7.52 | 6.27 | −0.35 | −0.40 |
| 2,2’,3,5,5’- | 224.5 | −0.34726 | 0.09667 | 7.82 | 6.32 | 7.43 | 6.22 | 0.39 | 0.10 |
| 2,2’,3,5,6- | 224.5 | −0.34678 | 0.09634 | 7.40 | 6.06 | 7.43 | 6.22 | −0.03 | −0.16 |
| 2,2’,3,5’,6- | 224.5 | −0.34390 | 0.09255 | 7.19 | 5.92 | 7.51 | 6.27 | −0.32 | −0.35 |
| 2,2’,3,4’,5’- | 224.5 | −0.34157 | 0.09673 | 7.76 | 6.30 | 7.34 | 6.17 | 0.42 | 0.13 |
| 2,2’,3,4’,6’- | 224.5 | −0.34292 | 0.10112 | 7.40 | 6.04 | 7.22 | 6.11 | 0.18 | −0.07 |
| 2,2’,4,4’,5- | 224.5 | −0.34864 | 0.09267 | 7.95 | 6.41 | 7.58 | 6.30 | 0.37 | 0.11 |
| 2,2’,4,4’,6- | 224.5 | −0.34908 | 0.09510 | 7.66 | 6.23 | 7.50 | 6.27 | 0.16 | −0.04 |
| 2,2’,4,5,5’- | 224.5 | −0.34567 | 0.09264 | 6.65 | 7.54 | 6.28 | 0.37 | ||
| 2,2’,4,5’,6- | 224.5 | −0.34263 | 0.09516 | 7.47 | 6.11 | 7.41 | 6.21 | 0.06 | −0.10 |
| 2,3,3’,4,4’- | 224.5 | −0.34251 | 0.08561 | 7.52 | 6.79 | 7.72 | 6.37 | −0.20 | 0.42 |
| 2,3,3’,4,5- | 224.5 | −0.34496 | 0.08289 | 7.68 | 6.92 | 7.84 | 6.44 | −0.16 | 0.48 |
| 2,3,3’,4’,6- | 224.5 | −0.34592 | 0.08472 | 7.65 | 6.20 | 7.80 | 6.42 | −0.15 | −0.22 |
| 2,3,3’,5,6- | 224.5 | −0.35128 | 0.08237 | 7.95 | 6.41 | 7.95 | 6.50 | 0.00 | −0.09 |
| 2,3,3’,5’,6- | 224.5 | −0.34429 | 0.08794 | 7.76 | 6.45 | 7.67 | 6.35 | 0.09 | 0.10 |
| 2,3,4,4’,5- | 224.5 | −0.34871 | 0.09230 | 7.50 | 6.71 | 7.59 | 6.31 | −0.09 | 0.40 |
| 2,3,4,4’,6- | 224.5 | −0.33919 | 0.08172 | 7.96 | 6.44 | 7.80 | 6.41 | 0.16 | 0.03 |
| 2,3,4’,5,6- | 224.5 | −0.34518 | 0.08471 | 7.88 | 6.39 | 7.79 | 6.41 | 0.09 | −0.02 |
| 2,3’,4,4’,5- | 224.5 | −0.34360 | 0.08771 | 7.33 | 6.57 | 7.67 | 6.35 | −0.34 | 0.22 |
| 2,3’,4,4’,6- | 224.5 | −0.34027 | 0.08002 | 7.91 | 6.40 | 7.87 | 6.45 | 0.04 | −0.05 |
| 2,3’,4,5,5’- | 224.5 | −0.34174 | 0.08062 | 6.30 | 7.87 | 6.45 | −0.15 | ||
| 2,3’,4,5’,6- | 224.5 | −0.34715 | 0.09151 | 7.92 | 6.42 | 7.59 | 6.31 | 0.33 | 0.11 |
| 2,3’,4,4’,5’- | 224.5 | −0.34973 | 0.09068 | 7.42 | 6.64 | 7.66 | 6.35 | −0.24 | 0.29 |
| 2,2’,3,3’,4,4’- | 237.4 | −0.35717 | 0.08854 | 6.96 | 8.21 | 6.71 | 0.25 | ||
| 2,2’,3,3’,4,5- | 237.4 | −0.35284 | 0.08620 | 8.42 | 6.76 | 8.22 | 6.71 | 0.20 | 0.05 |
| 2,2’,3,3’,4,5’- | 237.4 | −0.35195 | 0.08719 | 7.30 | 8.17 | 6.69 | 0.61 | ||
| 2,2’,3,3’,4,6- | 237.4 | −0.34963 | 0.08771 | 8.48 | 6.78 | 8.12 | 6.66 | 0.36 | 0.12 |
| 2,2’,3,3’,4,6’- | 237.4 | −0.35075 | 0.09200 | 7.65 | 6.20 | 8.00 | 6.60 | −0.35 | −0.40 |
| 2,2’,3,3’,5,5’- | 237.4 | −0.35253 | 0.08590 | 6.72 | 8.22 | 6.72 | 0.00 | ||
| 2,2’,3,3’,5,6- | 237.4 | −0.34916 | 0.08788 | 7.65 | 6.20 | 8.11 | 6.65 | −0.46 | −0.45 |
| 2,2’,3,3’,5,6’- | 237.4 | −0.34856 | 0.09087 | 7.82 | 6.32 | 8.00 | 6.60 | −0.18 | −0.28 |
| 2,2’,3,3’,6,6’- | 237.4 | −0.34611 | 0.09568 | 6.96 | 7.81 | 6.50 | 0.46 | ||
| 2,2’,3,4,4’,5- | 237.4 | −0.35384 | 0.08494 | 8.52 | 6.82 | 8.27 | 6.74 | 0.25 | 0.08 |
| 2,2’,3,4,4’,5’- | 237.4 | −0.35173 | 0.08704 | 8.38 | 6.73 | 8.17 | 6.69 | 0.21 | 0.04 |
| 2,2’,3,4,4’,6’- | 237.4 | −0.35560 | 0.09050 | 8.24 | 6.58 | 8.12 | 6.66 | 0.12 | −0.08 |
| 2,2’,3,4,5,5’- | 237.4 | −0.34844 | 0.08494 | 8.42 | 6.75 | 8.20 | 6.70 | 0.22 | 0.05 |
| 2,2’,3,4,5,6’- | 237.4 | −0.35021 | 0.09052 | 8.13 | 6.56 | 8.04 | 6.62 | 0.09 | −0.06 |
| 2,2’,3,4,5’,6- | 237.4 | −0.34545 | 0.08651 | 8.01 | 6.45 | 8.10 | 6.65 | −0.09 | −0.20 |
| 2,2’,3,4’,5,5’- | 237.4 | −0.35230 | 0.08574 | 8.58 | 6.85 | 8.23 | 6.72 | 0.35 | 0.13 |
| 2,2’,3,4’,5’,6- | 237.4 | −0.34866 | 0.09070 | 7.94 | 6.41 | 8.01 | 6.60 | −0.07 | −0.19 |
| 2,2’,3,5,5’,6- | 237.4 | −0.34523 | 0.08657 | 7.93 | 6.42 | 8.09 | 6.64 | −0.16 | −0.22 |
| 2,2’,4,4’,5,5’- | 237.4 | −0.35172 | 0.08561 | 8.49 | 6.80 | 8.22 | 6.71 | 0.27 | 0.09 |
| 2,2’,4,4’,5,6’- | 237.4 | −0.34981 | 0.08924 | 8.12 | 6.65 | 8.07 | 6.64 | 0.05 | 0.01 |
| 2,2’,4,4’,6,6’- | 237.4 | −0.35885 | 0.09295 | 8.12 | 6.54 | 8.09 | 6.65 | 0.03 | −0.11 |
| 2,3,3’,4,4’,5- | 237.4 | −0.34708 | 0.07635 | 8.31 | 7.44 | 8.46 | 6.83 | −0.15 | 0.61 |
| 2,3,3’,4,4’,6- | 237.4 | −0.34978 | 0.08301 | 8.48 | 6.78 | 8.28 | 6.74 | 0.20 | 0.04 |
| 2,3,3’,4’,5,6- | 237.4 | −0.34945 | 0.08314 | 8.48 | 6.78 | 8.27 | 6.74 | 0.21 | 0.04 |
| 2,3,3’,4’,5’,6- | 237.4 | −0.35415 | 0.08765 | 8.27 | 6.63 | 8.19 | 6.70 | 0.08 | −0.07 |
| 2,3,3’,5,5’,6- | 237.4 | −0.35226 | 0.08243 | 7.00 | 8.33 | 6.77 | 0.23 | ||
| 2,3’,4,4’,5,5’- | 237.4 | −0.34670 | 0.07271 | 8.21 | 7.29 | 8.57 | 6.89 | −0.36 | 0.40 |
| 3,3’,4,4’,5,5’- | 237.4 | −0.34085 | 0.06119 | 8.85 | 7.55 | 8.86 | 7.04 | −0.01 | 0.51 |
| 2,2’,3,3’,4,4’,5- | 250.3 | −0.35707 | 0.08042 | 8.90 | 7.08 | 8.84 | 7.11 | 0.06 | −0.03 |
| 2,2’,3,3’,4,4’,6- | 250.3 | −0.35814 | 0.08285 | 7.11 | 8.77 | 7.08 | 0.03 | ||
| 2,2’,3,3’,4,5,5’- | 250.3 | −0.35537 | 0.07930 | 9.10 | 7.21 | 8.85 | 7.12 | 0.25 | 0.09 |
| 2,2’,3,3’,4,5,6’- | 250.3 | −0.35338 | 0.08477 | 8.59 | 6.85 | 8.64 | 7.01 | −0.05 | −0.16 |
| 2,2’,3,3’,4,5’,6- | 250.3 | −0.35238 | 0.08192 | 8.68 | 6.92 | 8.72 | 7.05 | −0.04 | −0.13 |
| 2,2’,3,3’,4,6,6’- | 250.3 | −0.35462 | 0.08300 | 8.15 | 6.55 | 8.72 | 7.05 | −0.57 | −0.50 |
| 2,2’,3,3’,4,5’,6’ | 250.3 | −0.35079 | 0.08562 | 8.42 | 6.73 | 8.57 | 6.97 | −0.15 | −0.24 |
| 2,2’,3,3’,5,5’,6- | 250.3 | −0.35207 | 0.08211 | 8.59 | 6.85 | 8.71 | 7.04 | −0.12 | −0.19 |
| 2,2’,3,3’,5,6,6’- | 250.3 | −0.34986 | 0.08556 | 7.94 | 6.41 | 8.56 | 6.96 | −0.62 | −0.55 |
| 2,2’,3,4,4’,5,5’- | 250.3 | −0.35515 | 0.07911 | 9.10 | 7.21 | 8.85 | 7.12 | 0.25 | 0.09 |
| 2,2’,3,4,4’,5,6- | 250.3 | −0.35418 | 0.07794 | 8.97 | 7.13 | 8.88 | 7.13 | 0.09 | 0.00 |
| 2,2’,3,4,4’,5,6’- | 250.3 | −0.35507 | 0.08347 | 8.68 | 6.92 | 8.71 | 7.04 | −0.03 | −0.12 |
| 2,2’,3,4,4’,5’,6- | 250.3 | −0.35243 | 0.08170 | 8.85 | 7.04 | 8.73 | 7.05 | 0.12 | −0.01 |
| 2,2’,3,4,5,5’,6- | 250.3 | −0.34792 | 0.07777 | 8.75 | 6.99 | 8.79 | 7.08 | −0.04 | −0.09 |
| 2,2’,3,4’,5,6,6’- | 250.3 | −0.35225 | 0.08459 | 8.49 | 6.78 | 8.63 | 7.00 | −0.14 | −0.22 |
| 2,3,3’,4,4’,5,5’- | 250.3 | −0.35350 | 0.06968 | 8.72 | 7.72 | 9.14 | 7.27 | −0.42 | 0.45 |
| 2,3,3’,4,4’,5,6- | 250.3 | −0.35197 | 0.07472 | 8.91 | 7.08 | 8.95 | 7.17 | −0.04 | −0.09 |
| 2,3,3’,4,4’,5’,6- | 250.3 | −0.35930 | 0.07838 | 9.10 | 7.21 | 8.94 | 7.17 | 0.16 | 0.04 |
| 2,3,3’,4,5,5’,6- | 250.3 | −0.35485 | 0.07407 | 9.10 | 7.21 | 9.01 | 7.20 | 0.09 | 0.01 |
| 2,3,3’,4’,5,5’,6- | 250.3 | −0.35800 | 0.07853 | 9.10 | 7.21 | 8.91 | 7.15 | 0.19 | 0.06 |
| 2,2’,3,3’,4,4’,5,5’- | 263.2 | −0.36003 | 0.07389 | 9.70 | 7.62 | 9.46 | 7.51 | 0.24 | 0.11 |
| 2,2’,3,3’,4,4’,5,6- | 263.2 | −0.36014 | 0.07477 | 9.29 | 7.35 | 9.43 | 7.50 | −0.14 | −0.15 |
| 2,2’,3,3’,4,4’,5,6’- | 263.2 | −0.35760 | 0.07749 | 9.42 | 7.43 | 9.31 | 7.43 | 0.11 | 0.00 |
| 2,2’,3,3’,4,4’,6,6’- | 263.2 | −0.35828 | 0.08100 | 9.10 | 7.21 | 9.20 | 7.38 | −0.10 | −0.17 |
| 2,2’,3,3’,4,5,5’,6- | 263.2 | −0.35470 | 0.07375 | 9.42 | 7.43 | 9.39 | 7.47 | 0.03 | −0.04 |
| 2,2’,3,3’,4,5,5’,6’- | 263.2 | −0.35688 | 0.07765 | 9.10 | 7.21 | 9.29 | 7.42 | −0.19 | −0.21 |
| 2,2’,3,3’,4,5,6,6’- | 263.2 | −0.35309 | 0.07686 | 9.20 | 7.30 | 9.26 | 7.41 | −0.06 | −0.11 |
| OctaCl- | |||||||||
| 2,2’,3,3’,4,5’,6,6’- | 263.2 | −0.35469 | 0.08120 | 9.29 | 7.35 | 9.14 | 7.35 | 0.15 | 0.00 |
| 2,2’,3,4,4’,5,5’,6- | 263.2 | −0.35469 | 0.07336 | 9.50 | 7.49 | 9.40 | 7.48 | 0.10 | 0.01 |
| 2,2’,3,4,4’,5,6,6’- | 263.2 | −0.35822 | 0.07589 | 9.48 | 7.48 | 9.37 | 7.47 | 0.11 | 0.01 |
| 2,3,3’,4,4’,5,5’,6- | 263.2 | −0.36143 | 0.07507 | 9.70 | 7.62 | 9.44 | 7.51 | 0.26 | 0.11 |
| 2,2’,3,3’,4,4’,5,5’,6- | 276.1 | −0.35975 | 0.07059 | 10.18 | 7.94 | 9.93 | 7.83 | 0.25 | 0.11 |
| 2,2’,3,3’,4,4’,5,6,6’- | 276.1 | −0.36005 | 0.07278 | 10.07 | 7.88 | 9.87 | 7.80 | 0.20 | 0.08 |
| 2,2’,3,3’,4,5,5’,6,6’- | 276.1 | −0.35684 | 0.07291 | 8.20 | 9.81 | 7.77 | 0.43 | ||
| DecaCl- | 289.0 | −0.36182 | 0.07013 | 8.20 | 10.35 | 8.12 | 0.08 | ||
| Chloronaphthalenes | |||||||||
| 1- | 143.1 | −0.29516 | 0.08950 | 4.24 | 4.23 | 0.01 | |||
| 2- | 143.1 | −0.29795 | 0.09062 | 4.14 | 4.24 | −0.10 | |||
| 1,2- | 156.0 | −0.30423 | 0.07999 | 4.42 | 4.74 | −0.32 | |||
| 1,4- | 156.0 | −0.30258 | 0.07629 | 4.66 | 4.79 | −0.13 | |||
| 1,5- | 156.0 | −0.30286 | 0.07636 | 4.67 | 4.79 | −0.12 | |||
| 1,7- | 156.0 | −0.30507 | 0.07757 | 4.56 | 4.79 | −0.23 | |||
| 1,8- | 156.0 | −0.29812 | 0.07750 | 4.41 | 4.73 | −0.32 | |||
| 2,3- | 156.0 | −0.30553 | 0.07967 | 4.71 | 4.75 | −0.04 | |||
| 2,7- | 156.0 | −0.30912 | 0.07857 | 4.81 | 4.80 | 0.01 | |||
| 1,3,7- | 168.9 | −0.31340 | 0.06630 | 5.35 | 5.31 | 0.04 | |||
| 2,3,6- | 168.9 | −0.31512 | 0.06818 | 5.12 | 5.30 | −0.18 | |||
| 1,2,3,4- | 181.8 | −0.31569 | 0.05984 | 5.75 | 5.71 | 0.04 | |||
| 1,2,3,5- | 181.8 | −0.31696 | 0.05772 | 5.77 | 5.75 | 0.02 | |||
| 1,3,5,7- | 181.8 | −0.31919 | 0.05479 | 6.19 | 5.82 | 0.37 | |||
| 1,3,5,8- | 181.8 | −0.31355 | 0.05442 | 5.76 | 5.78 | −0.02 | |||
| 1,4,6,7- | 181.8 | −0.31813 | 0.05561 | 5.81 | 5.80 | 0.01 | |||
| Chlorobenzenes | |||||||||
| Mono- | 102.2 | −0.33466 | 0.13195 | 2.98 | 3.00 | −0.02 | |||
| 1,2- | 113.1 | −0.34231 | 0.11787 | 3.38 | 3.53 | −0.15 | |||
| 1,3- | 114.6 | −0.34436 | 0.11598 | 3.48 | 3.61 | −0.13 | |||
| 1,4- | 115.2 | −0.33830 | 0.11560 | 3.38 | 3.58 | −0.20 | |||
| 1,2,3- | 125.3 | −0.35227 | 0.10497 | 4.04 | 4.08 | −0.04 | |||
| 1,2,4- | 128.1 | −0.34649 | 0.10290 | 3.98 | 4.13 | −0.15 | |||
| 1,3,5- | 128.1 | −0.35843 | 0.10131 | 4.02 | 4.26 | −0.24 | |||
| 1,2,3,4- | 141.0 | −0.35244 | 0.09274 | 4.55 | 4.62 | −0.07 | |||
| 1,2,3,5- | 141.0 | −0.35558 | 0.09100 | 4.65 | 4.67 | −0.02 | |||
| 1,2,4,5- | 141.0 | −0.35147 | 0.09104 | 4.51 | 4.64 | −0.13 | |||
| Penta- | 153.9 | −0.35809 | 0.08139 | 5.03 | 5.12 | −0.09 | |||
| Hexa- | 166.8 | −0.36423 | 0.07241 | 5.47 | 5.59 | −0.12 | |||
| 3,4-Dimethyl- | 131.6 | −0.31901 | 0.13266 | 3.82 | 3.46 | 0.36 | |||
| Chlorotoluenes | |||||||||
| 2- | 116.9 | −0.32902 | 0.13290 | 3.42 | 3.24 | 0.18 | |||
| 3- | 118.1 | −0.32981 | 0.13103 | 3.28 | 3.30 | −0.02 | |||
| 4- | 118.3 | −0.32396 | 0.13090 | 3.33 | 3.26 | 0.07 | |||
| 2,4- | 129.0 | −0.33516 | 0.11725 | 4.24 | 3.81 | 0.43 | |||
| 2,6- | 126.9 | −0.34055 | 0.11804 | 4.29 | 3.80 | 0.49 | |||
| 2,3-diCl-p-cymene | 190.0 | −0.32996 | 0.12206 | 5.50 | 4.94 | 0.56 | |||
| 2,5-diCl-p-cymene | 190.0 | −0.32714 | 0.11994 | 5.60 | 4.95 | 0.65 | |||
| 2,3,6-triCl-p-cymene | 202.9 | −0.33672 | 0.10871 | 6.20 | 5.49 | 0.71 | |||
| TetraCl-p-cymene | 215.8 | −0.34333 | 0.09615 | 6.80 | 6.02 | 0.78 | |||
| Bromobenzenes | |||||||||
| Mono | 105.5 | −0.33082 | 0.12952 | 3.02 | 3.08 | −0.06 | |||
| 1,2- | 121.6 | −0.33684 | 0.11453 | 3.64 | 3.72 | −0.08 | |||
| 1,3- | 121.6 | −0.33898 | 0.11280 | 3.75 | 3.76 | −0.01 | |||
| 1,4- | 121.6 | −0.33232 | 0.11143 | 3.79 | 3.73 | 0.06 | |||
| 1,3,5- | 137.7 | −0.35159 | 0.09877 | 4.51 | 4.44 | 0.07 | |||
| 1,2,4,5- | 153.8 | −0.34276 | 0.08639 | 5.13 | 4.91 | 0.22 | |||
| Hexa- | 186.0 | −0.35176 | 0.06184 | 5.73 | 6.06 | −0.33 | |||
| Bromotoluenes | |||||||||
| 2- | 120.2 | −0.32632 | 0.13018 | 3.42 | 3.33 | 0.09 | |||
| 3- | 121.3 | −0.32684 | 0.12998 | 3.28 | 3.36 | −0.08 | |||
| 4- | 122.5 | −0.32096 | 0.12962 | 3.33 | 3.34 | −0.01 | |||
| Bromochlorobenzenes | |||||||||
| 2- | 116.8 | −0.33903 | 0.11593 | 3.83 | 3.61 | 0.22 | |||
| 4- | 116.8 | −0.33494 | 0.11346 | 3.83 | 3.62 | 0.21 | |||
| Descriptor | V | EHOMO | ELUMO |
|---|---|---|---|
| S | 0.0033 | 3.2308 | 4.2833 |
| t-score | 9.0395 | −3.4612 | −7.6414 |
| Significance | 0.0000 | 0.0007 | 0.0000 |
| V | EHOMO | ELUMO | |
|---|---|---|---|
| V | 1 | ||
| EHOMO | −0.5981 | 1 | |
| ELUMO | −0.5704 | 0.2004 | 1 |
| Substitution patterns | logPOW exp.a | logPOW calc.b | logPOW CLogPc | ΔlogPOWd | ΔlogPOWe |
|---|---|---|---|---|---|
| Chlorobiphenyls | |||||
| 2- | 4.38 | 4.55 | 4.49 | −0.17 | −0.11 |
| 4- | 4.63 | 4.67 | 4.74 | −0.04 | −0.11 |
| 2,2’- | 4.72 | 4.89 | 4.96 | −0.17 | −0.24 |
| 2,3- | 4.99 | 5.02 | 5.09 | −0.03 | −0.10 |
| 2,6- | 4.93 | 4.97 | 4.96 | −0.04 | −0.03 |
| 4,4’- | 5.23 | 5.17 | 5.46 | 0.06 | −0.23 |
| 2,2’,4- | 5.39 | 5.40 | 5.67 | −0.01 | −0.28 |
| 2,2’,5- | 5.33 | 5.37 | 5.67 | −0.04 | −0.34 |
| 2,3,4- | 5.68 | 5.49 | 5.68 | 0.19 | 0.00 |
| 2,3,4’- | 5.29 | 5.49 | 5.80 | −0.20 | −0.51 |
| 2,4,6- | 5.50 | 5.53 | 5.67 | −0.03 | −0.17 |
| 2,2’,3,3’- | 5.67 | 5.80 | 6.14 | −0.13 | −0.47 |
| 2,2’,3,5’- | 5.73 | 5.80 | 6.26 | −0.07 | −0.53 |
| 2,2’,4,4’- | 5.94 | 5.88 | 6.38 | 0.06 | −0.44 |
| 2,3,4,5- | 6.05 | 5.98 | 6.39 | 0.07 | −0.34 |
| 2,3,5,6- | 5.96 | 5.97 | 6.26 | −0.01 | −0.30 |
| 3,3’,4,4’- | 6.21 | 6.12 | 6.64 | 0.09 | −0.43 |
| 2,2’,3,3’,6- | 5.60 | 6.19 | 6.60 | −0.59 | −1.00 |
| 2,2’,3,4,4’- | 6.18 | 6.30 | 6.85 | −0.12 | −0.67 |
| 2,2’,3,5,5’- | 6.32 | 6.22 | 6.97 | 0.10 | −0.65 |
| 2,3,4,4’,5- | 6.71 | 6.31 | 7.10 | 0.40 | −0.39 |
| 2,3,4,4’,6- | 6.44 | 6.41 | 6.97 | 0.03 | −0.53 |
| 2,2’,3,4,4’,5- | 6.82 | 6.74 | 7.57 | 0.08 | −0.75 |
| 2,3,3’,4,4’,5- | 7.44 | 6.83 | 7.70 | 0.61 | −0.26 |
| 2,3,3’,4,4’,6- | 6.78 | 6.74 | 7.57 | 0.04 | −0.79 |
| 2,2’,3,3’,4,5,5’- | 7.21 | 7.12 | 8.16 | 0.09 | −0.95 |
| 2,2’,3,4,4’,5,6- | 7.13 | 7.13 | 8.15 | 0.00 | −1.02 |
| 2,2’,3,4,5,5’,6- | 6.99 | 7.08 | 8.15 | −0.09 | −1.16 |
| 2,3,3’,4,4’,5,5’- | 7.72 | 7.27 | 8.29 | 0.45 | −0.57 |
| 2,2’,3,3’,4,4’,5,5’- | 7.62 | 7.51 | 8.75 | 0.11 | −1.13 |
| 2,2’,3,3’,4,4’,5,6- | 7.35 | 7.50 | 8.62 | −0.15 | −1.27 |
| 2,2’,3,3’,4,5’,6,6’- | 7.35 | 7.35 | 8.49 | 0.00 | −1.14 |
| 2,2’,3,3’,4,4’,5,5’,6- | 7.94 | 7.83 | 9.34 | 0.11 | −1.40 |
| 2,2’,3,3’,4,4’,5,6,6’- | 7.88 | 7.80 | 9.21 | 0.08 | −1.33 |
| Deca- | 8.20 | 8.12 | 9.92 | 0.08 | −1.72 |
| Chloronapthalenes | |||||
| 2- | 4.14 | 4.24 | 4.03 | −0.10 | 0.11 |
| 1,4- | 4.66 | 4.79 | 4.74 | −0.13 | −0.08 |
| 1,7- | 4.56 | 4.79 | 4.74 | −0.23 | −0.18 |
| 2,3- | 4.71 | 4.75 | 4.62 | −0.04 | 0.09 |
| 2,3,6- | 5.12 | 5.30 | 5.34 | −0.18 | −0.22 |
| 1,2,3,5- | 5.77 | 5.75 | 5.93 | 0.02 | −0.16 |
| 1,4,6,7- | 5.81 | 5.80 | 6.05 | 0.01 | −0.24 |
| Chlorobenzenes | |||||
| 1,2- | 3.38 | 3.53 | 3.45 | −0.15 | −0.07 |
| 1,2,3- | 4.04 | 4.08 | 4.04 | −0.04 | 0.00 |
| 1,3,5- | 4.02 | 4.26 | 4.28 | −0.24 | −0.26 |
| 1,2,3,4- | 4.55 | 4.62 | 4.63 | −0.07 | −0.08 |
| Penta- | 5.03 | 5.12 | 5.35 | −0.09 | −0.32 |
| Hexa- | 5.47 | 5.59 | 6.06 | −0.12 | −0.59 |
| 3,4-Dimethyl- | 3.82 | 3.46 | 3.80 | 0.36 | 0.02 |
| Chlorotoluenes | |||||
| 2- | 3.42 | 3.24 | 3.35 | 0.18 | 0.07 |
| 2,6- | 4.29 | 3.80 | 4.07 | 0.49 | 0.22 |
| 2,5-diCl-p-cymene | 5.60 | 4.95 | 5.49 | 0.65 | 0.11 |
| Bromobenzenes | |||||
| Mono- | 3.02 | 3.08 | 3.01 | −0.06 | 0.01 |
| 1,3- | 3.75 | 3.76 | 3.87 | −0.01 | −0.12 |
| Hexa- | 5.73 | 6.06 | 6.72 | −0.33 | −0.99 |
| 4-Cl- | 3.83 | 3.62 | 3.72 | 0.21 | 0.11 |
| Bromotoluenes | |||||
| 4- | 3.33 | 3.34 | 3.50 | −0.01 | −0.17 |
Share and Cite
Gao, S.; Cao, C. A New Approach on Estimation of Solubility and n-Octanol/ Water Partition Coefficient for Organohalogen Compounds. Int. J. Mol. Sci. 2008, 9, 962-977. https://doi.org/10.3390/ijms9060962
Gao S, Cao C. A New Approach on Estimation of Solubility and n-Octanol/ Water Partition Coefficient for Organohalogen Compounds. International Journal of Molecular Sciences. 2008; 9(6):962-977. https://doi.org/10.3390/ijms9060962
Chicago/Turabian StyleGao, Shuo, and Chenzhong Cao. 2008. "A New Approach on Estimation of Solubility and n-Octanol/ Water Partition Coefficient for Organohalogen Compounds" International Journal of Molecular Sciences 9, no. 6: 962-977. https://doi.org/10.3390/ijms9060962




