Synthesis and Characterization of N,N’-(propane-1,2 diyldicarbamothioyl)dibenzamide

Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Xu, X.Y.; Qian, X.H.; Li, Z.; Huang, Q.C.; Chen, G. J. Fluorine Chem. 2003, 121, 51–54. [CrossRef]
- Zhou, W.Q.; Yang, W.; Qiu, L.H.; Zhang, Y.; Yu, Z.F. J. Mol. Struct. 2005, 749, 89–95.
- Arslan, H.; Külcü, N.; Flörke, U. Spectrochim. Acta Pt. A 2006, 64, 1065–1071. [CrossRef] [PubMed]
- Kodomari, M.; Suzuki, M.; Tanigawa, K.; Aoyama, T. Tetrahedron Lett. 2005, 46, 5841–5843.
- Binzet, G.; Arslan, H.; Flörke, U.; Külcü, N.; Duran, N. J. Coordination Chem. 2006, 59, 1395–1406. [CrossRef]
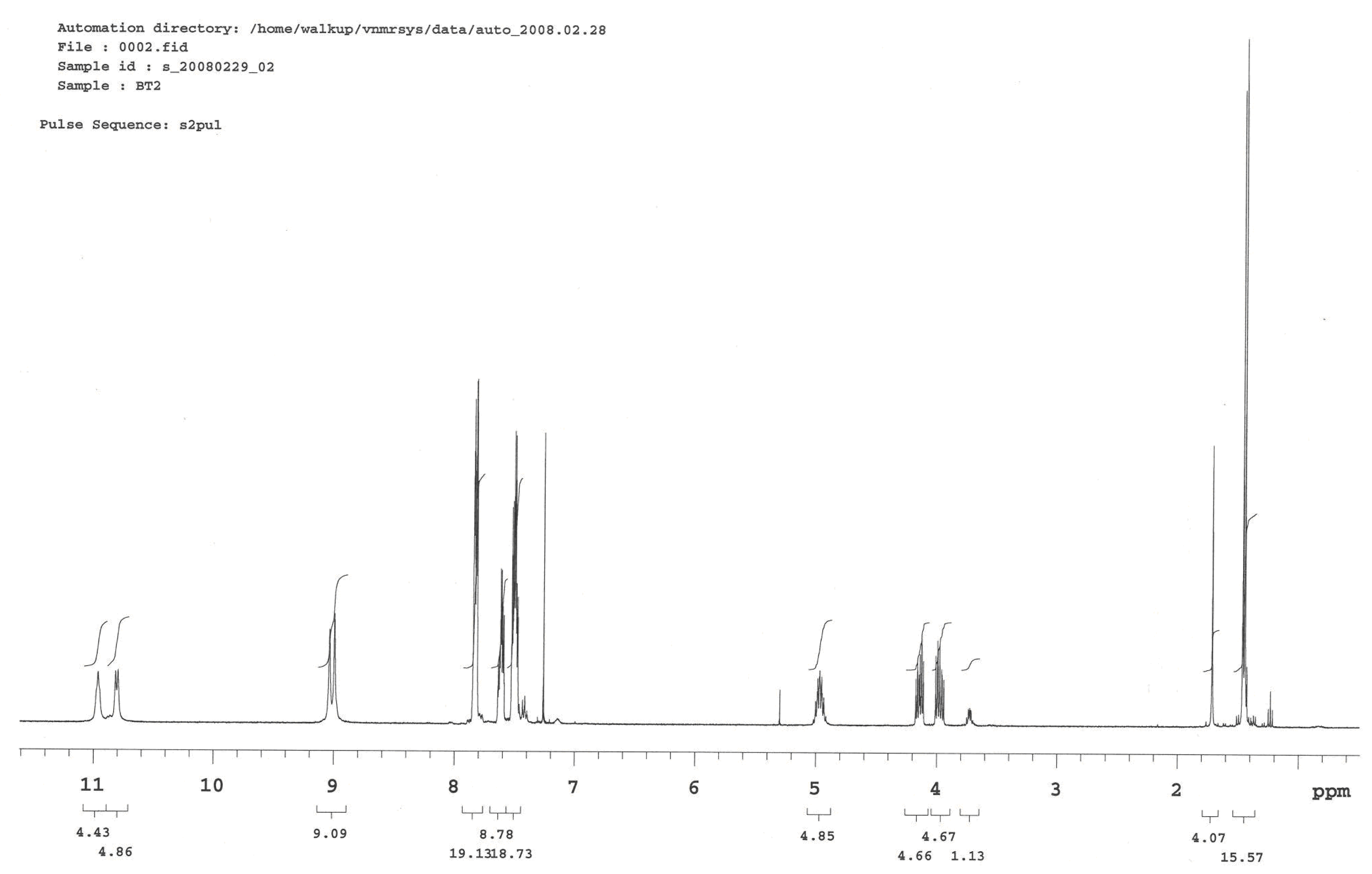
| Sample | Stage | TG results temperature range (0C) | DTA results temperature peak (°C) | Weight loss (%) Found/Calculated | Evolved moiety |
| 2 | I | 180–240 | 226.71 | 43.684/44.75 | C6H5CONHCSNH |
| II | 240–340 | 282.56 | 44.884/44.75 | C6H5CONHCSNH |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kurt, G.; Mercimek, B. Synthesis and Characterization of N,N’-(propane-1,2 diyldicarbamothioyl)dibenzamide. Molbank 2008, 2008, M578. https://doi.org/10.3390/M578
Kurt G, Mercimek B. Synthesis and Characterization of N,N’-(propane-1,2 diyldicarbamothioyl)dibenzamide. Molbank. 2008; 2008(3):M578. https://doi.org/10.3390/M578
Chicago/Turabian StyleKurt, Gülşah, and Bedrettin Mercimek. 2008. "Synthesis and Characterization of N,N’-(propane-1,2 diyldicarbamothioyl)dibenzamide" Molbank 2008, no. 3: M578. https://doi.org/10.3390/M578




