Synthesis and Characterization of a Novel 2-Pyrazoline
Abstract
:Introduction
Results and Discussion
Experimental
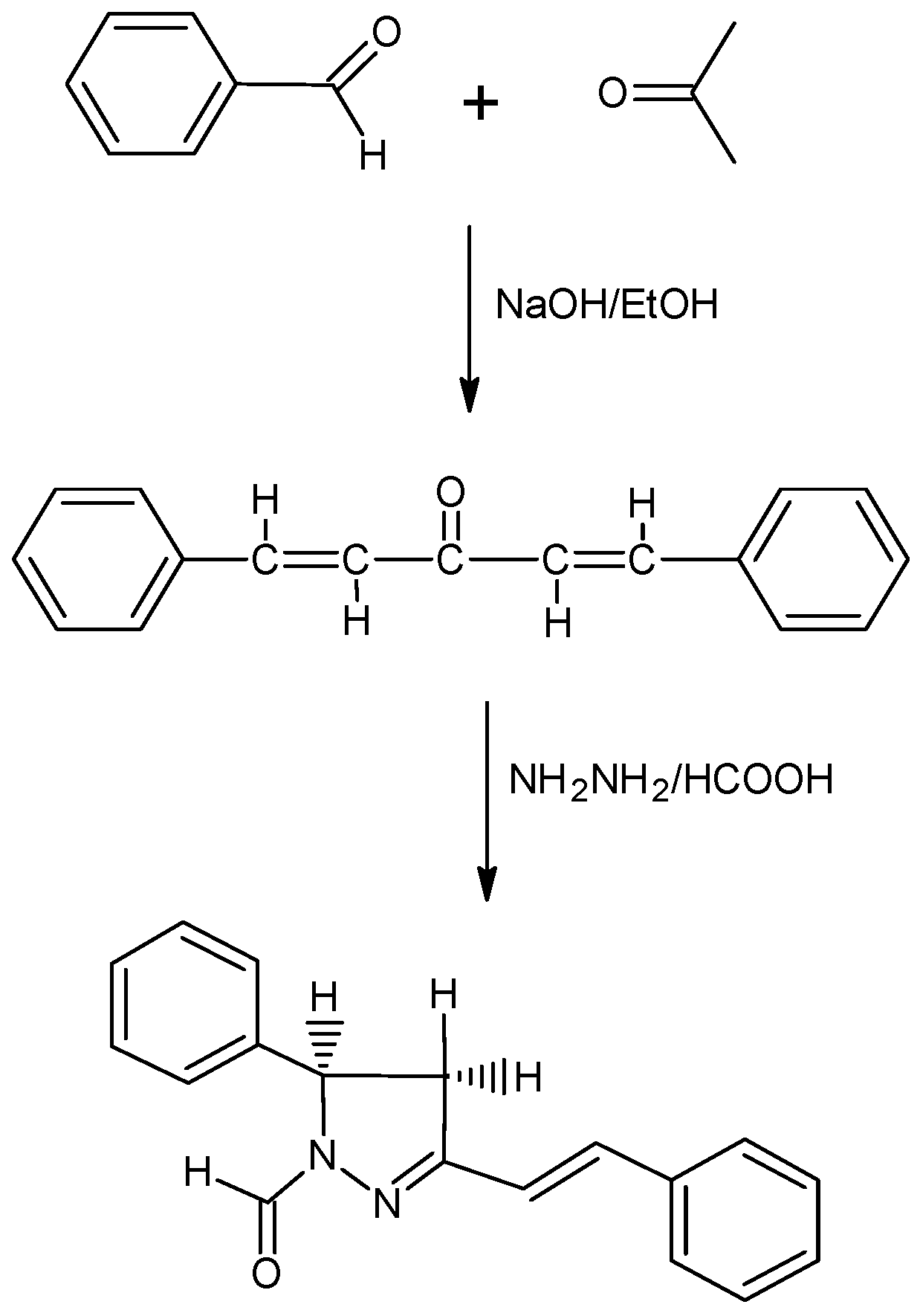
Preparation of dibenzalacetone
Preparation of 5-phenyl-3-[(E)-2-phenylvinyl]-4,5-dihydro-1H-pyrazole-1-carbaldehyde
Conclusions
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
References
- Zhang, X.H.; Wu, S.K.; Gao, Z.Q.; Lee, C.S.; Lee, S.T.; Kwong, H.L. Thin Solid Films. 2000, 371, 40–46. [CrossRef]
- Ramalingham, K.; Thyvekikakath, G.X.; Berlin, K.D.; Chesnut, R.W.; Brown, R.A.; Durham, N.N.; Ealick, A.E.; Vender, H.D. J. Med. Chem. 1977, 20, 847.
- Korgaokar, S.S.; Patil, P.H.; Shah, M.J.; Parekh, H.H. Indian J. Pharm. Sci. 1996, 58, 222–225.
- Rajendra, P.Y.; Lakshmana, R.A.; Prasoona, L.; Murali, K.; Ravi, K.P. Bioorg. Med. Chem. Lett. 2005, 15, 5030–5034.
- Lombardino, J.G.; Otterness, I.G. J. Med. Chem. 1981, 24, 830. [CrossRef] [PubMed]
- Ozdemir, Z.; Kandilici, H.B.; Gumusel, B.; Calis, U.; Bilgin, A.A. Eur. J. Med. Chem. 2007, 42, 373–379. [PubMed]
- Taylor, E.C.; Patel, H.H. Tetrahedron. 1992, 48, 8089–8100. [CrossRef]
- Budakoti, A.; Abid, M.; Azam, A. Eur. J. Med. Chem. 2006, 41, 63–70. [CrossRef] [PubMed]
- Zitouni, G.T.; Ozdemir, A.; Guven, K. Arch. Pharm. (Weinheim). 2005, 338, 96–104.
- Fathalla, O.A.; Zaki, M.E.; Swelam, S.A.; Nofal, S.M.; El-Eraky, W.I. Acta Pol. Pharm. 2003, 60, 51–60. [PubMed]
- Levai, A. Monatsh. Chem. 1995, 126, 1245–1251.
- Ali, M.A.; Siddiqui, M.S.A.A. Eur. J. Med. Chem. 2007, 42, 268–275. [CrossRef] [PubMed]
- Amir, M.; Kumar, H.; Khan, S.A. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [CrossRef] [PubMed]
- Budakoti, A.; Abid, M.; Azam, A. Eur. J. Med. Chem. 2007, 42, 544–551. [CrossRef] [PubMed]
- Pinto, D.C.G.A.; Silva, A.M.S.; Cavaleiro, J.A.S.; Foces-Foces, C.; Llamas- Saiz, A.L.; Jagerovic, N.; Elguero, J. Tetrahedron. 1999, 55, 10187. [CrossRef]
- Toth, G.; Levai, A.; Dinya, Z.; Snatzke, G. Tetrahedron. 1991, 47, 8119–81132.
- Mustafa, A.; Hilmy, M.K. J. Chem. Soc. 1951, 3254.
- Toth, G.; Szolosy, A.; Levai, A.; Kotovych, G. J. Chem. Soc. Perkin Trans. 2 1986, 1895.
- Toth, G.; Levai, A.; Duddeck, H. Mag. Reson. Chem. 1992, 30, 235.
- Toth, G.; Levai, A.; Szolosy, A.; Duddeck, H. Tetrahedron. 1993, 49, 863.
- Kamecki, J.; Perka, W.; Pijewska, J. Polish. J. Chem. 1985, 59, 285.
- Pijewska, L.; Kamecki, J.; Perka-Karolczak, W. Pharmzie. 1993, 48, 254.
- Bhanumati, S.; Deep, H. Indian J. Chem. 2006, 45, 2353.
- Ali, A.K.; Kamal, I.A.; Ismail, A.M. J. Macromol. Sci.-Pure Appl. Chem. 2002, 39, 333–350.
- Levai, A. J. Heterocyclic Chem. 2002, 39, 1.
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Text Book of Practical Organic Chemistry, International Student Eddition ed; Addition Wesley Longman: England, 1989; Vol. 6, p. 1033. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Singh, P.; Negi, J.S.; Nee Pant, G.J.; Rawat, M.S.M.; Budakoti, A. Synthesis and Characterization of a Novel 2-Pyrazoline. Molbank 2009, 2009, M614. https://doi.org/10.3390/M614
Singh P, Negi JS, Nee Pant GJ, Rawat MSM, Budakoti A. Synthesis and Characterization of a Novel 2-Pyrazoline. Molbank. 2009; 2009(3):M614. https://doi.org/10.3390/M614
Chicago/Turabian StyleSingh, Pramod, Jagmohan S. Negi, Geeta Joshi Nee Pant, Mohan S. M. Rawat, and Asha Budakoti. 2009. "Synthesis and Characterization of a Novel 2-Pyrazoline" Molbank 2009, no. 3: M614. https://doi.org/10.3390/M614



