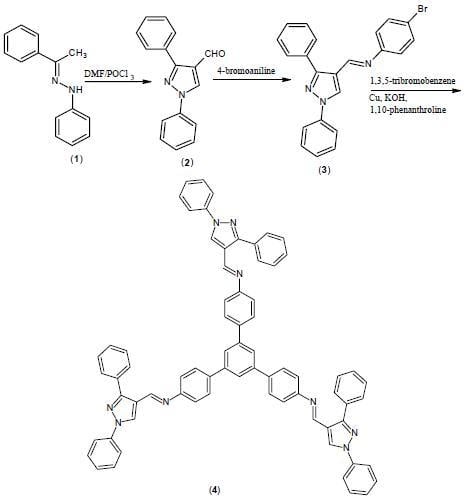
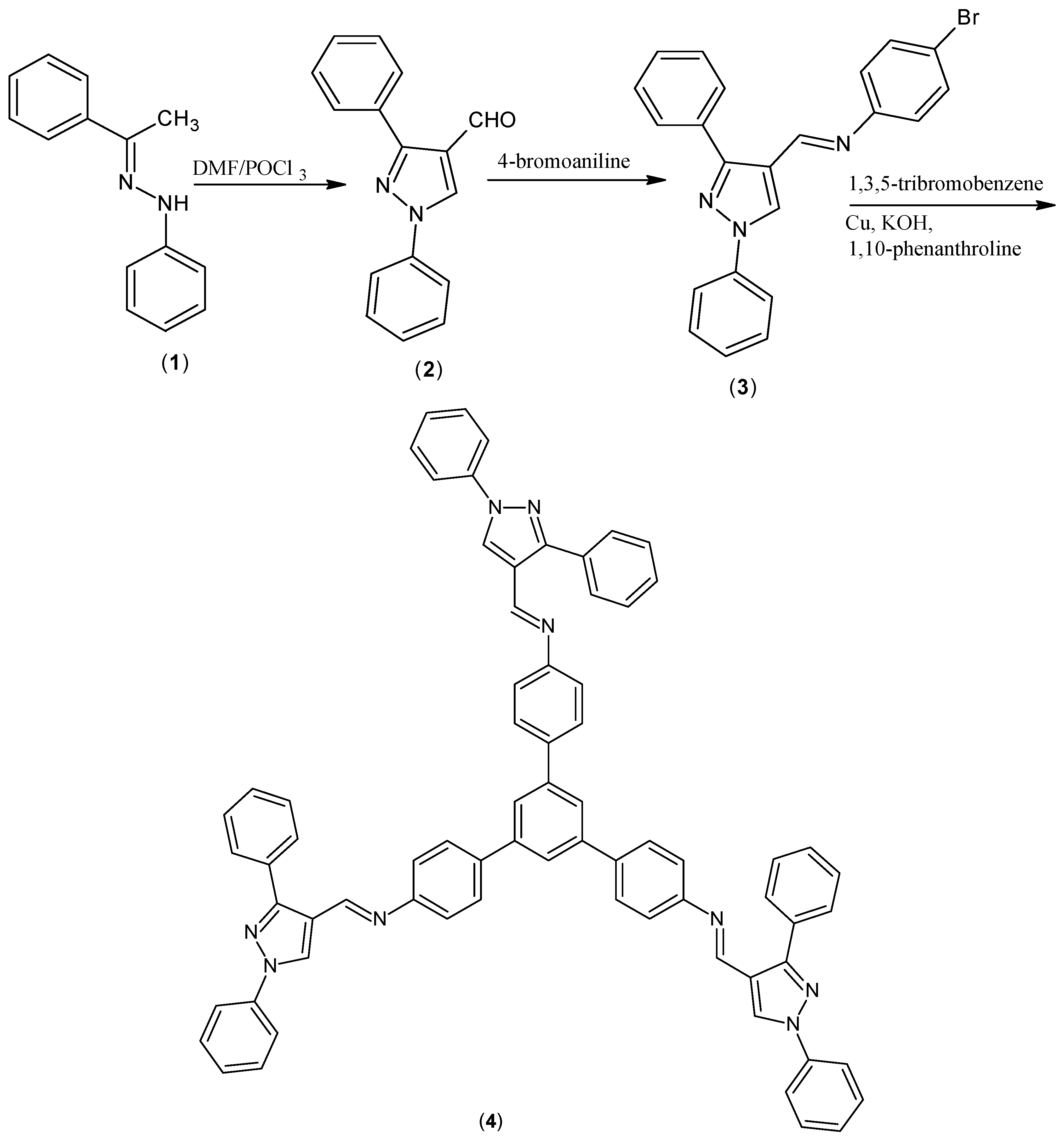
1,3,5-Tris{[N-(1,3-diphenyl-1H-pyrazol-4-yl)methylene]-4-aminophenyl}benzene
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Thelakkat, M.; Schmitz, C.; Hohle, C.; Strohriegl, P.; Schmidt, H.-W.; Hofmann, U.; Schloter, S.; Haarer, D. Novel functional materials based on triarylamines-synthesis and application in electroluminescent devices and photorefractive systems. Phys. Chem. Chem. Phys. 1999, 1, 1693–1698. [Google Scholar] [CrossRef]
- Mohite, S.K.; Magdum, C.S. Novel synthesis of functionally substituted N-{[3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl]methylene}anilines and their pharmacological screening. Int. J. Chem. Sci. 2006, 4, 980–988. [Google Scholar]
- Rathelot, P.; Azas, N.; El-Kashef, H.; Delmas, F.; Di Giorgio, C.; Timon-David, P.; Maldonado, J.; Vanelle, P. 1,3-Diphenylpyrazoles: Synthesis and antiparasitic activities of azomethine derivatives. Eur. J. Med. Chem. 2002, 37, 671–679. [Google Scholar] [CrossRef]
- Goodbrand, H.B.; Hu, N.-X. Ligand-accelerated catalysis of the Ullmann condensation: Application to hole conducting triarylamines. J. Org. Chem. 1999, 64, 670–674. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Liebeskind, L.S. Ambient temperature, Ullmann-like reductive coupling of aryl, heteroaryl, and alkenyl halides. J. Org. Chem. 1997, 62, 2312–2313. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Veettil, S.P.; Haridas, K.R. 1,3,5-Tris{[N-(1,3-diphenyl-1H-pyrazol-4-yl)methylene]-4-aminophenyl}benzene. Molbank 2009, 2009, M624. https://doi.org/10.3390/M624
Veettil SP, Haridas KR. 1,3,5-Tris{[N-(1,3-diphenyl-1H-pyrazol-4-yl)methylene]-4-aminophenyl}benzene. Molbank. 2009; 2009(4):M624. https://doi.org/10.3390/M624
Chicago/Turabian StyleVeettil, Sandhya P., and Karickal R. Haridas. 2009. "1,3,5-Tris{[N-(1,3-diphenyl-1H-pyrazol-4-yl)methylene]-4-aminophenyl}benzene" Molbank 2009, no. 4: M624. https://doi.org/10.3390/M624