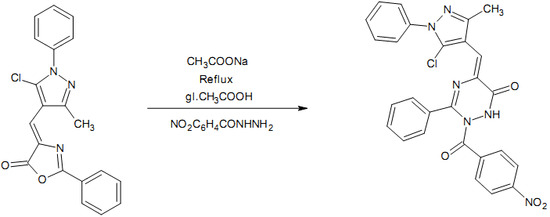
5-[(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-nitrobenzoyl)-3-phenyl-2,5-dihydro-1,2,4-triazin-6(1H)-one
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
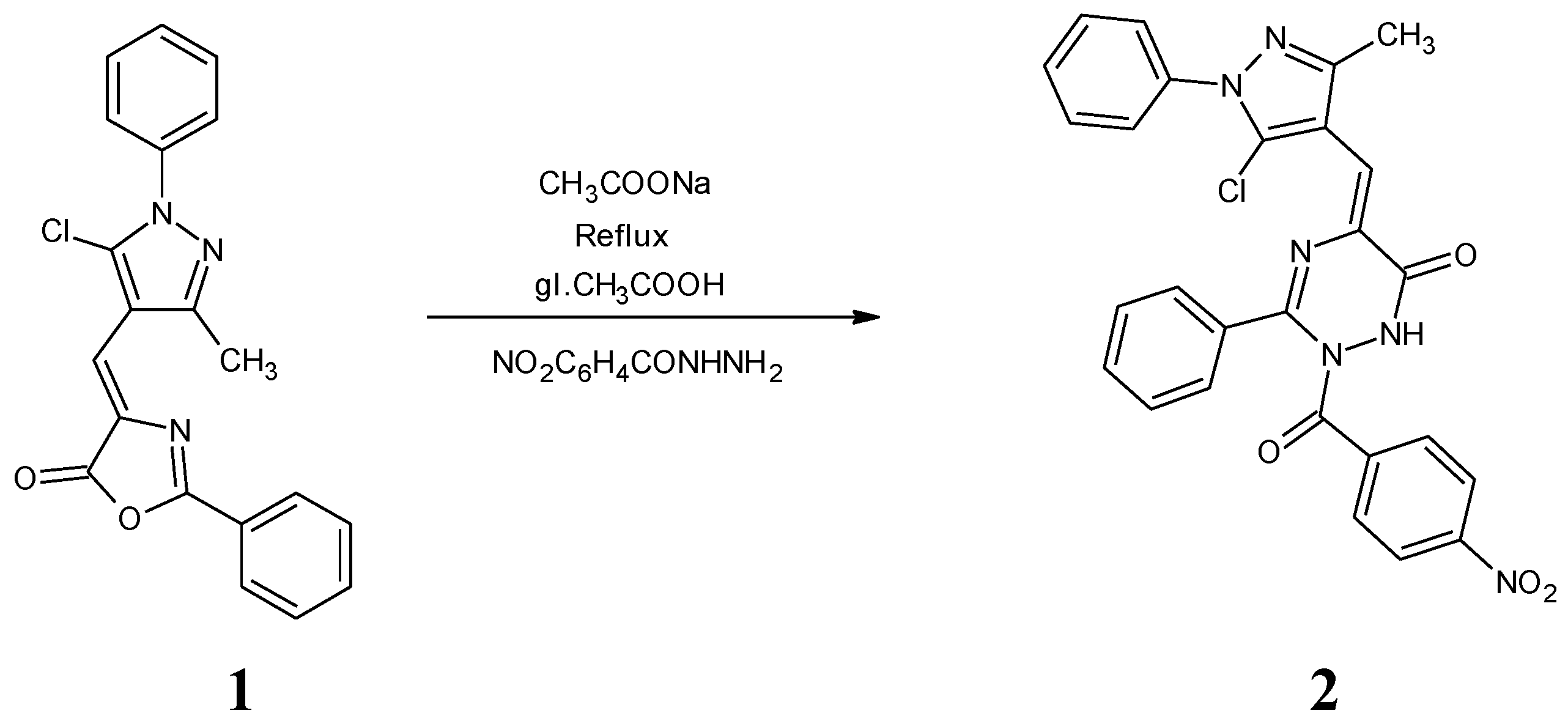
Synthesis of 5-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-nitrobenzoyl)-3-phenyl-1, 2-dihydro-1, 2, 4-triazin-6(5H)-one (2)
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
References and Notes
- Boger, D.L. Diels-Alder cycloaddition reactions of heterocyclic azadienes: Scope and applications. Chem. Rev. 1986, 86, 781–793. [Google Scholar] [CrossRef]
- Boger, D.L. Diels-Alder reactions of azadienes. Tetrahedron 1983, 39, 2869–2939. [Google Scholar] [CrossRef]
- Neunhoeffer, H. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; p. 385. [Google Scholar]
- Yuen, A.W. Lamotrigine: A review of antiepileptic efficacy. Epilepsia 1994, 35, S33–S36. [Google Scholar] [CrossRef]
- Kaushik, D.; Khan, S.A.; Chawla, G. Design & synthesis of 2-(substituted aryloxy)-5-(substituted benzylidene)-3-phenyl-2, 5-dihydro-1H-[1, 2, 4] triazin-6-one as potential anticonvulsant agents. Eur. J. Med. Chem. 2010, 45, 3960–3969. [Google Scholar] [PubMed]
- Gibson, N.W.; Erickson, L.C.; Hickman, J.A. Effects of the antitumor agent 8-carbamoyl-3-(2-chloroethyl) imidazo [5, 1-d]-1, 2, 3, 5-tetrazin-4(3H)-one on the DNA of mouse L1210 cells. Cancer Res. 1984, 44, 1767–1771. [Google Scholar] [PubMed]
- Smith, R.H.; Scudiero, D.A.; Michejda, C.J. 1, 3-Dialkyl-3-acyltriazenes, a novel class of antineoplastic alkylating agents. J. Med. Chem. 1990, 33, 2579–2583. [Google Scholar] [CrossRef] [PubMed]
- Sztanke, K.; Rzymowska, J.; Niemczyk, M.; Dybała, I.; Kozioł, A.E. Synthesis, crystal structure and anticancer activity of novel derivatives of ethyl 1-(4-oxo-8-aryl-4,6,7,8 tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl)formate. Eur. J. Med. Chem. 2006, 41, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Sztanke, K.; Pasternak, K.; Sztanke, M.; Kandefer-Szerszen, M.; Kozioł, A.E.; Dybała, I. Crystal structure, antitumour and antimetastatic activities of disubstituted fused 1,2,4-triazinones. Bioorg. Med. Chem. Lett. 2009, 19, 5095–5100. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Husain, K.; Athar, F.; Azam, A. Synthesis and antiamoebic activity of 3, 7- dimethyl-pyrazolo [3, 4-e][1,2,4] triazin-4-yl thiosemicarbazide derivatives. Eur. J. Pharma. Sci. 2005, 25, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.M.; Saeda, M.; Fawzy, M.; El-Baz, M. Synthesis of some new 1, 6-dihydro-3-substituted 6-spiro-(9'-fluorene)-1, 2, 4-triazin-5-(4H)-ones as potential anti HIV and anticancer drugs. Pharmazie 1994, 49, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.B.; Srivastava, K.; Puri, S.K.; Chauhan, P.M.S. Synthesis of 2-[3, 5- Substituted Pyrazol-1-yl]-4, 6-trisubstituted triazine derivatives as antimalarial agents. Bioorg. Med. Chem. Lett. 2005, 15, 4957–4960. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; Srinivas, U.; Jayathirtha, R.; Bhanuprakash, K.; Harakishore, K.; Murthy, U.S.N. Synthesis and antibacterial activity of 2,4,6-trisubstituted s-triazines. Bioorg. Med. Chem. Lett. 2005, 15, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Jha, A.K.; Thakur, A.S.; Dewangan, D. Synthesis and antibacterial activity of some 1, 3, 4-oxadiazole derivatives and their thione analogues. Int. J. Res. Pharma. Bio. Sci. 2011, 2, 215–219. [Google Scholar]
- Saravanan, J.; Mohan, S.; Roy, J.J. Synthesis of some 3-substituted amino-4,5-tetramethylene thieno[2,3-d][ 1,2,3]-triazin-4(3H)-ones as potential antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 4365–4369. [Google Scholar] [CrossRef] [PubMed]
- Sangshetti, J.N.; Shinde, D.B. One pot synthesis and SAR of some novel 3-substituted 5,6-diphenyl-1,2,4-triazines as antifungal agents. Bioorg. Med. Chem. Lett. 2010, 20, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.C.A.; Briggs, E.; Clarke, E.D.; Whittingham, W.G. Synthesis and SAR studies of novel antifungal 1,2,3-triazines. Bioorg. Med. Chem. Lett. 2007, 17, 5222–5226. [Google Scholar] [CrossRef] [PubMed]
- Dawane, B.S.; Kadam, S.N.; Shaikh, B.M. An efficient synthesis of 1, 2, 4-triazine derivatives and their in vitro antimicrobial activity. Der. Pharmacia Lett. 2010, 2, 126–131. [Google Scholar]
- Yang, R.; Kaplan, P.A. Reaction of isothiourea with 2,3-diaza-3-pentenedioic anhydride: A solid-phase synthesis of 3-amino-1,2,4-triazin-5(4H)-ones. Tetrahedron Lett. 2001, 42, 4433–4435. [Google Scholar] [CrossRef]
- Oettmeier, W.; Hilp, U.; Draber, W. Structure-activity relationships of triazinone herbicides on resistant weeds and resistant chlamydomonas reinhardtii. Pestic. Soc. 1991, 33, 399–409. [Google Scholar] [CrossRef]
- Kranz, E.; Santel, H.; Luerssen, K. New 6-cyclo-butyl-1, 2, 4-triazinone derivatives—useful as herbicides and plant growth regulators. DE: 3917043 A1, 1990. [Google Scholar]
- Kaushik, D.; Verma, T.; Madaan, K. 2-(Benzoylamino)-3-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)acrylic acid. Molbank 2011, 2011, M726. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaushik, D.; Verma, T. 5-[(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-nitrobenzoyl)-3-phenyl-2,5-dihydro-1,2,4-triazin-6(1H)-one. Molbank 2011, 2011, M733. https://doi.org/10.3390/M733
Kaushik D, Verma T. 5-[(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-nitrobenzoyl)-3-phenyl-2,5-dihydro-1,2,4-triazin-6(1H)-one. Molbank. 2011; 2011(3):M733. https://doi.org/10.3390/M733
Chicago/Turabian StyleKaushik, Darpan, and Tarawanti Verma. 2011. "5-[(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-nitrobenzoyl)-3-phenyl-2,5-dihydro-1,2,4-triazin-6(1H)-one" Molbank 2011, no. 3: M733. https://doi.org/10.3390/M733