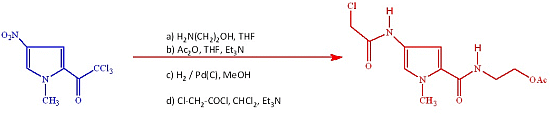
2-(4-(2-Chloroacetamido)-1-methyl-1H-pyrrole-2-carboxamido)ethyl Acetate
Abstract
:Introduction
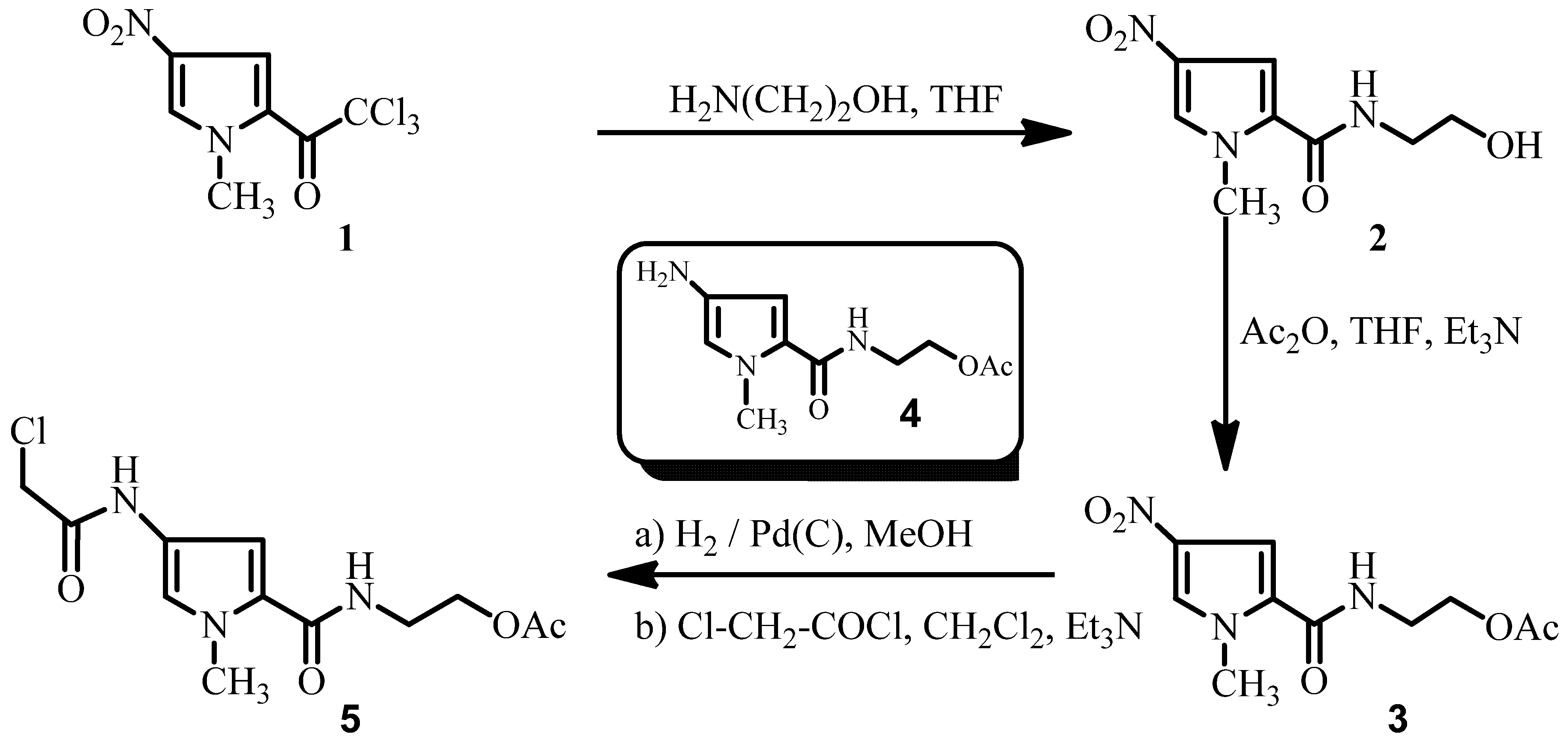
Results and Discussion
Experimental
2-(4-(2-Chloroacetamido)-1-methyl-1H-pyrrole-2-carboxamido)ethyl Acetate
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Conflict of Interest
References
- Zimmer, C. Effects of the antibiotics netropsin and distamycin A on the structure and function of nucleic acids. Prog. Nucleic Acid Res. Mol. Biol. 1975, 15, 285–291. [Google Scholar] [PubMed]
- Lown, J.W. Lexitropsins in antiviral drug development. Antiviral Res. 1992, 17, 179–185. [Google Scholar] [CrossRef]
- Arcamone, F.; Penco, S.; Orezzi, P.; Nicolella, V.; Pirelli, A. Structure and synthesis of distamycin. Nature 1964, 203, 1064–1065. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.S.; Shah, A.; Rehman, Z.U.; Barker, D. Chemistry of DNA minor groove binding agents. J. Photochem. Photobiol. B 2012, 115, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Iaconinoto, M.A.; Carrion, M.D.; Tabrizi, M.; A. Gambari, R.; Borgatti, M.; Heilmann, J. Synthesis and biological activity of alpha-bromoacryloyl lexitropsin conjugates. Eur. J. Med. Chem. 2005, 40, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Al-Said, N.H.; Shawakfeh, K.Q. Synthetic studies towards bis-heterodimeric netropsin analogs. Jordan J. Chem. 2007, 2, 125–132. [Google Scholar]
- Al-Said, N.H.; Klaib, S. Synthesis of a C-2 stapled bis-lexitropsin. Monatsh. Chem. 2007, 138, 573–577. [Google Scholar] [CrossRef]
- Al-Said, N.H. Synthesis of a terminally linked homodimeric bis-distamycin analog. Monatsh. Chem. 2006, 137, 1535–1541. [Google Scholar] [CrossRef]
- Zhao, R.; Al-Said, N.H.; Sternbach, D.L.; Lown, J.W. Camptothecin and minor-groove binder hybrid molecules: Synthesis, inhibition of topoisomerase I, and anticancer cytotoxicity in vitro. J. Med. Chem. 1997, 40, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Al-Said, N.H.; Oreski, B.; Lown, J.W. Design, synthesis and topoisomerase II inhibition activity of 4'-demethylepipodopyllotoxine-lexitropsin conjugate. Anticancer Drug Des. 1996, 11, 325–338. [Google Scholar] [PubMed]
- Al-Said, N.H.; Lown, J.W. A convenient synthesis of cross-linked homodimeric lexitropsins. Synth. Comm. 1995, 25, 1059–1070. [Google Scholar] [CrossRef]
- Belanger, P. Electrophilic substitutions on 2-trichloroacetylpyrrole. Tetrahedron Lett. 1979, 27, 2505–2508. [Google Scholar] [CrossRef]
- Rahman, K.M.; Jackson, P.J.M.; James, C.J.; Basu, P.; Hartley, J.A.; de la Fuente, M.; Schatzlein, A.; Robson, M.; Pedley, R.B.; Pepper, C.; et al. GC-Targeted C8-linked pyrrolobenzodiazepine-biaryl conjugates with femtomolar in vitro cytotoxicity and in vivo antitumor activity in mouse models. J. Med. Chem. 2013, 56, 2911–2935. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.; Kennedy, A.R.; Suckling, C.J. A divergent synthesis of minor groove binders with tail group variation. Org. Biomol. Chem. 2009, 7, 178–186. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Said, N.H. 2-(4-(2-Chloroacetamido)-1-methyl-1H-pyrrole-2-carboxamido)ethyl Acetate. Molbank 2014, 2014, M820. https://doi.org/10.3390/M820
Al-Said NH. 2-(4-(2-Chloroacetamido)-1-methyl-1H-pyrrole-2-carboxamido)ethyl Acetate. Molbank. 2014; 2014(1):M820. https://doi.org/10.3390/M820
Chicago/Turabian StyleAl-Said, Naim H. 2014. "2-(4-(2-Chloroacetamido)-1-methyl-1H-pyrrole-2-carboxamido)ethyl Acetate" Molbank 2014, no. 1: M820. https://doi.org/10.3390/M820