Straightforward Synthesis of 2(5H)-Furanones as Promising Cross-Coupling Partners: Direct Furanone Annulation Utilizing Ti-Mediated Aldol Addition
Abstract
:1. Introduction
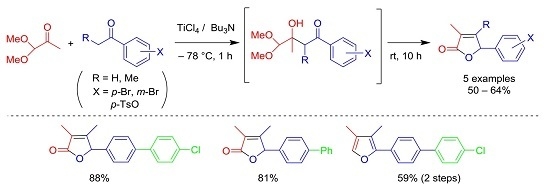
2. Results and Discussion
3. Experimental Section
3.1. General
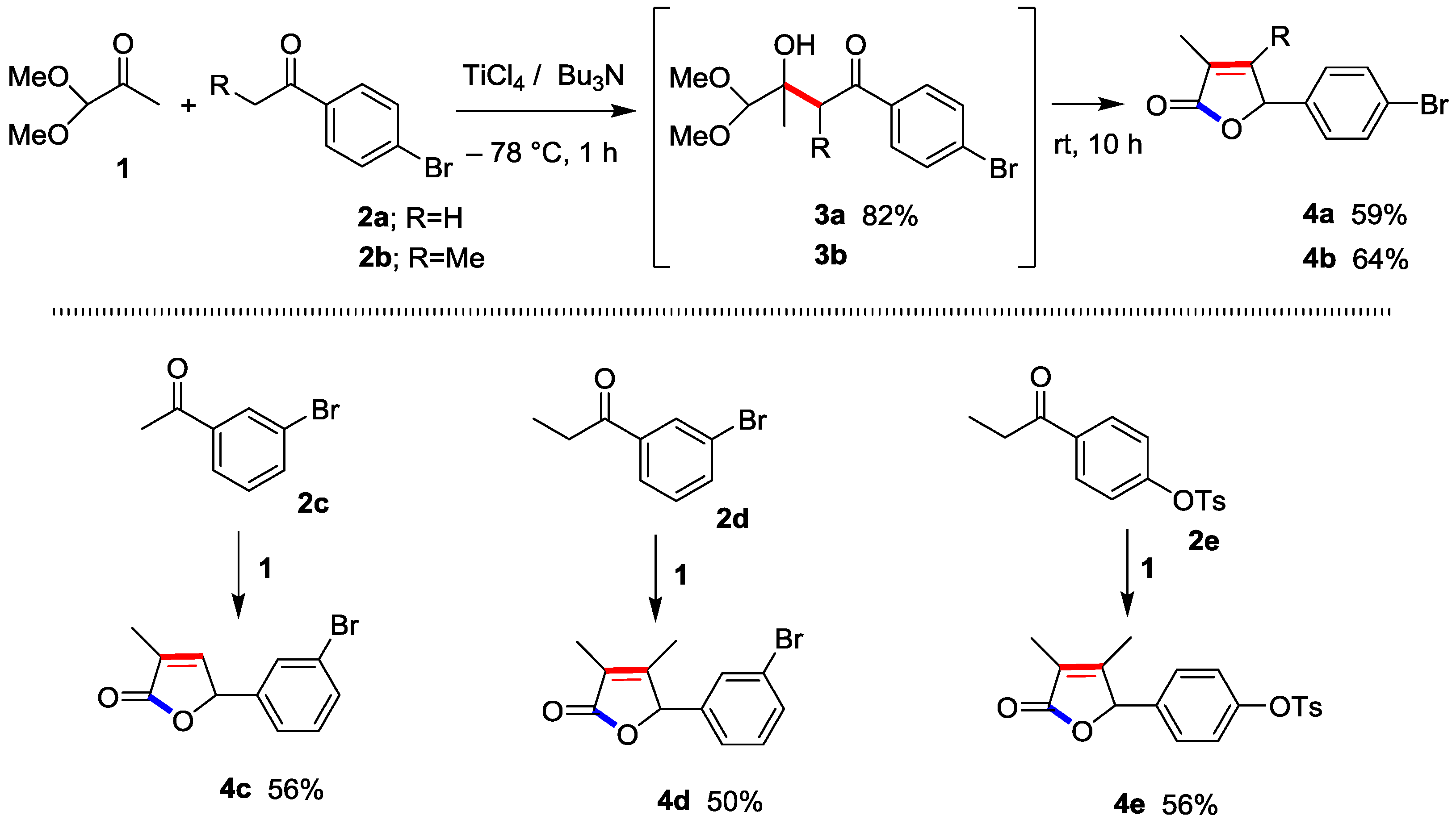
3.2. Preparation of 1-(4-Bromophenyl)-3-hydroxy-4,4-dimethoxy-3-methylbutan-1-one (3a)
3.3. Preparation of 5-(4-Bromophenyl)-3-methylfuran-2(5H)-one (4a) [4]
3.4. Preparation of 5-(4-Bromophenyl)-3,4-dimethylfuran-2(5H)-one (4b)
3.5. Preparation of 5-(3-Bromophenyl)-3-methylfuran-2(5H)-one (4c)
3.6. Preparation of 5-(3-Bromophenyl)-3,4-dimethylfuran-2(5H)-one (4d)
3.7. Preparation of 5-(4-Tosyloxyphenyl)-3,4-dimethylfuran-2(5H)-one (4e)
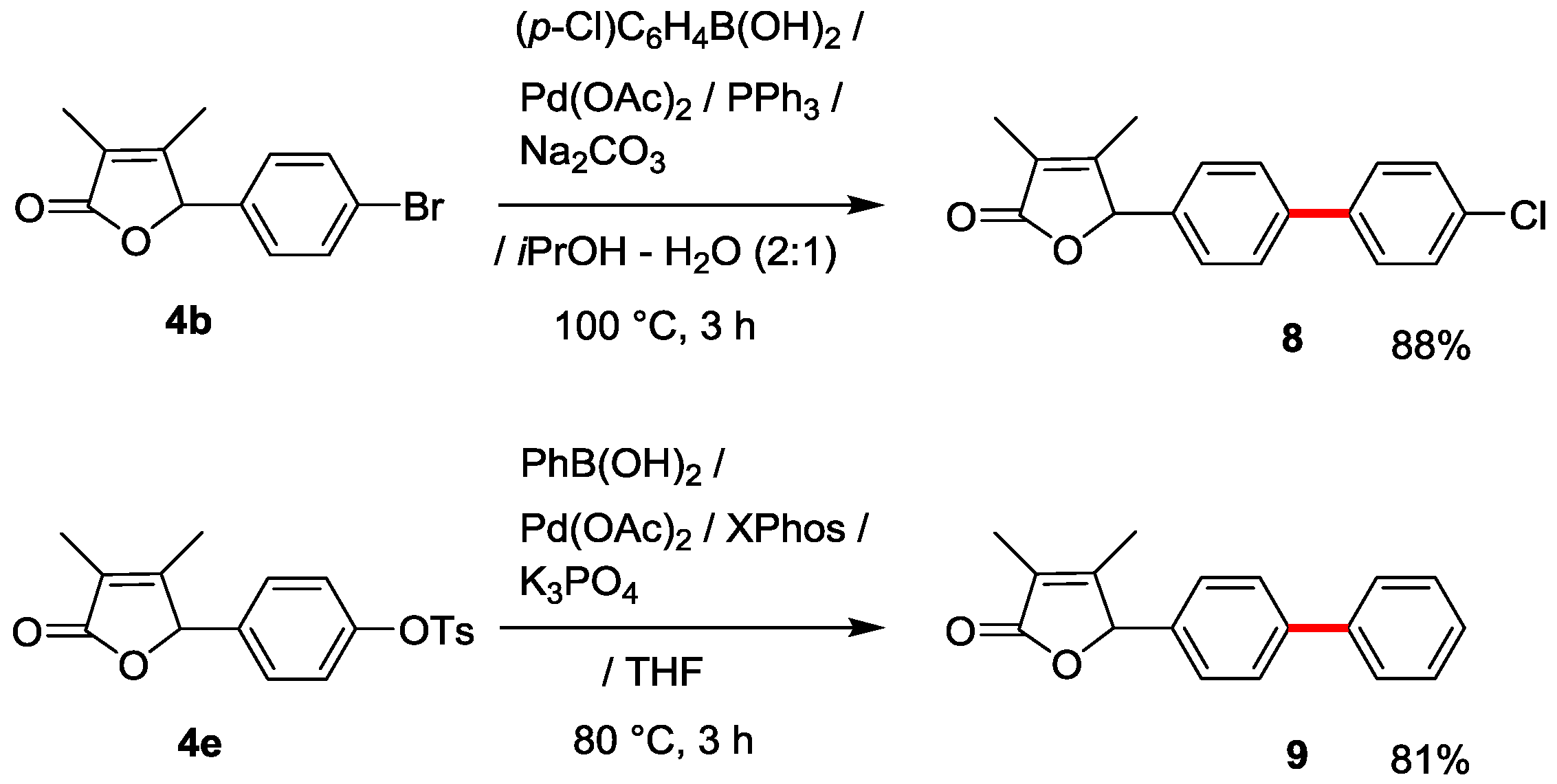
3.8. Synthesis of 5-(4’-Chloro-[1,1’-biphenyl]-4-yl)-3,4-dimethylfuran-2(5H)-one (8)
3.9. Synthesis of 5-(1,1’-Biphenyl-4-yl)-3,4-dimethylfuran-2(5H)-one (9) [25]
3.10. Synthesis of 2-(4-Bromopheyl)-3,4-dimethylfuran (10)
3.11. Synthesis of 2-(4’-Chloro-[1,1’-biphenyl]-4-yl)-3,4-dimethylfuran (11)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marshal, P.G. Rodd’s Chemistry of Carbon Compounds, 2nd ed.; Elsevier: New York, NY, USA, 1970; Volume 2, Part D; pp. 369–375. [Google Scholar]
- Rao, Y.S. Recent advances in the chemistry of unsaturated lactones. Chem. Rev. 1976, 76, 625–694. [Google Scholar] [CrossRef]
- Knight, D.W. Synthetic approaches to butenolides. Contemp. Org. Synth. 1994, 1, 287–315. [Google Scholar] [CrossRef]
- Yoneda, E.; Zhang, S.W.; Zhou, D.Y.; Onitsuka, K.; Takahashi, S. Ruthenium-catalyzed cyclocarbonylation of allenyl alcohols and amines: Selective synthesis of lactones and lactams. J. Org. Chem. 2003, 68, 8571–8576. [Google Scholar] [CrossRef] [PubMed]
- He, R.J.; Zhu, B.C.; Wang, Y.G. Lewis base-catalyzed electrophilic lactonization of selenyl bromide resin and facile solid-phase synthesis of furan-2(5H)-one derivatives. Appl. Organometal. Chem. 2014, 28, 523–528. [Google Scholar]
- Tanabe, Y. The selective Claisen and Dieckmann ester condensations promoted by dichlorobis(trifluoromethanesulfonato)titanium (IV). Bull. Chem. Soc. Jpn. 1989, 62, 1917–1924. [Google Scholar] [CrossRef]
- Tanabe, Y.; Hamasaki, R.; Funakoshi, S. Powerful Claisen condensation and Claisen-aldol tandem reaction of α,α-disubstituted esters promoted by ZrCl4‒iPr2NEt. Chem. Commun. 2001, 1674–1675. [Google Scholar] [CrossRef]
- Tanabe, Y.; Makita, A.; Funakoshi, S.; Hamasaki, R.; Kawakusu, T. Practical synthesis of Z-Civetone utilizing Ti-Dieckmann condensation. Adv. Synth. Catal. 2002, 344, 507–510. [Google Scholar] [CrossRef]
- Misaki, T.; Nagase, R.; Matsumoto, K.; Tanabe, Y. Ti-crossed Claisen condensation between carboxylic esters and acid chlorides or acids: Highly selective and general method for the preparation of various β-keto esters. J. Am. Chem. Soc. 2005, 127, 2854–2855. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Nakazawa, S.; Okabayashi, T.; Horii, A.; Misaki, T.; Tanabe, Y. Powerful Ti-crossed Claisen condensation between ketene silyl acetals or thioacetals and acid chlorides or acids. Org. Lett. 2006, 8, 5215–5218. [Google Scholar] [CrossRef] [PubMed]
- Nagase, R.; Oguni, Y.; Ureshino, S.; Mura, H.; Misaki, T.; Tanabe, Y. Asymmetric Ti-crossed Claisen condensation: Application to concise asymmetric total synthesis of Alternaric acid. Chem. Commun. 2013, 7001–7003. [Google Scholar] [CrossRef] [PubMed]
- Ashida, Y.; Kajimoto, S.; Nakatsuji, H.; Tanabe, Y. Synthesis of methyl 1-formylcyclopropanecarboxylate utilizing Ti-Claisen condensation. Org. Synth. Accepted paper.
- Tanabe, Y.; Matsumoto, N.; Higashi, T.; Misaki, T.; Itoh, T.; Yamamoto, M.; Mitarai, K.; Nishii, Y. Direct, practical, and powerful crossed-aldol additions between ketones and ketones or aldehydes utilizing environmentally benign TiCl4–Bu3N reagent. Tetrahedron (Symposium) 2002, 58, 8269–8280. [Google Scholar] [CrossRef]
- Nagase, R.; Matsumoto, N.; Hosomi, K.; Higashi, T.; Funakoshi, S.; Misaki, T.; Tanabe, Y. Ti-direct, powerful, stereoselective aldol-type additions of esters and thioesters to carbonyl compounds: Application to the synthesis and evaluation of lactone analogs of Jasmone perfumes. Org. Biomol. Chem. 2007, 5, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Funatomi, T.; Nakazawa, S.; Matsumoto, K.; Nagase, R.; Tanabe, Y. Ti-mediated direct and highly stereoselective Mannich reactions between esters and oxime ethers. Chem. Commun. 2008, 771–773. [Google Scholar] [CrossRef]
- Tanabe, Y.; Ohno, N. A novel and efficient synthesis of 2(5H)-furanone derivative. J. Org. Chem. 1988, 53, 1560–1563. [Google Scholar] [CrossRef]
- Tanabe, Y.; Mitarai, K.; Higashi, T.; Misaki, T.; Nishii, Y. Efficient one-step synthesis of trialkylsubstituted 2(5H)-furanone utilizing direct Ti-crossed aldol condensation and its application to the straightforward synthesis of (R)-mintlactone and (R)-menthofuran. Chem. Commun. 2002, 2542–2543. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Iwasawa, N.; Stevens, R.W.; Haga, T. Synthetic control leading to chiral compounds. Tetrahedron 1984, 40, 1381. [Google Scholar] [CrossRef]
- Gao, P.; Xu, P.-F.; Zhai, H. Expeditious construction of (+)-mintlactone via intramolecular hetero-Pauson−Khand reaction. J. Org. Chem. 2009, 74, 2592–2593. [Google Scholar] [CrossRef] [PubMed]
- Curini, M.; Epifano, F.; Montanari, F. Oxone®-KI induced lactonization and etherification of unsaturated acids and alcohols: A formal synthesis of mintlactone. Synlett 2004, 368–370. [Google Scholar] [CrossRef]
- Bates, R.W.; Scidhar, S. A synthesis of (−)-Mintlactone. J. Org. Chem. 2008, 73, 8104–8105. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Mehta, G. A total synthesis of sarcandralactone A: A general, concise, RCM enabled approach to lindenanolide sesquiterpenoids. Tetrahedron Lett. 2015, 56, 3941–3944. [Google Scholar] [CrossRef]
- Ramesh, S.; Mehta, G. A general, concise, ‘collective’ approach to eudesmanolide sesquiterpenoids: total synthesis of bioactive atractylenolides I-IV and related natural products. Tetrahedron Lett. 2015, 56, 5545–5548. [Google Scholar] [CrossRef]
- Billingsley, K.; Buchwald, S.L. Highly efficient monophosphine-based catalyst for the palladium-catalyzed suzuki-miyaura reaction of heteroaryl halides and heteroaryl boronic acids and esters. J. Am. Chem. Soc. 2007, 129, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.E.; Couper, M. The reduction of cis and trans 2,3-dimethyl-3-p-xenoylacrylic acids and their esters. J. Org. Chem. 1941, 6, 91–104. [Google Scholar] [CrossRef]
- Sugi, M.; Nagase, R.; Misaki, T.; Nakatsuji, H.; Tanabe, Y. Asymmetric Total Synthesis of (–)-Azaspirene by utilizing Ti-Claisen Condensation and Ti-Direct Aldol Reaction. Eur. J. Org. Chem. in press. [CrossRef]

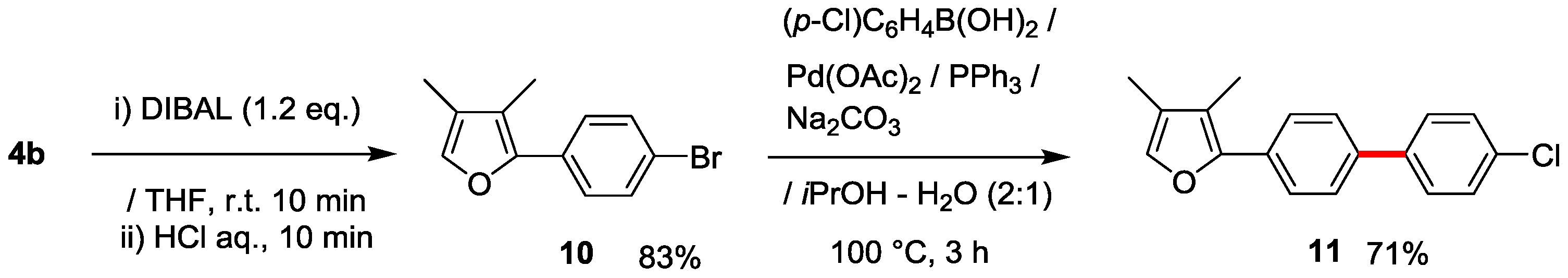
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, Y.; Ashida, Y.; Nakatsuji, H.; Tanabe, Y. Straightforward Synthesis of 2(5H)-Furanones as Promising Cross-Coupling Partners: Direct Furanone Annulation Utilizing Ti-Mediated Aldol Addition. Molbank 2016, 2016, M908. https://doi.org/10.3390/M908
Ban Y, Ashida Y, Nakatsuji H, Tanabe Y. Straightforward Synthesis of 2(5H)-Furanones as Promising Cross-Coupling Partners: Direct Furanone Annulation Utilizing Ti-Mediated Aldol Addition. Molbank. 2016; 2016(4):M908. https://doi.org/10.3390/M908
Chicago/Turabian StyleBan, Yuki, Yuichiro Ashida, Hidefumi Nakatsuji, and Yoo Tanabe. 2016. "Straightforward Synthesis of 2(5H)-Furanones as Promising Cross-Coupling Partners: Direct Furanone Annulation Utilizing Ti-Mediated Aldol Addition" Molbank 2016, no. 4: M908. https://doi.org/10.3390/M908