Dimethyl 7-(dimethylamino)-3,4-dihydro-1-(2-oxopropyl)-4-phenylnaphthalene-2,2(1H)-dicarboxylate
Abstract
:1. Introduction
2. Results
3. Experimental Section
3.1. General Information
3.1.1. Synthesis of (E)-4-(3-(Dimethylamino)phenyl)but-3-en-2-one (1)
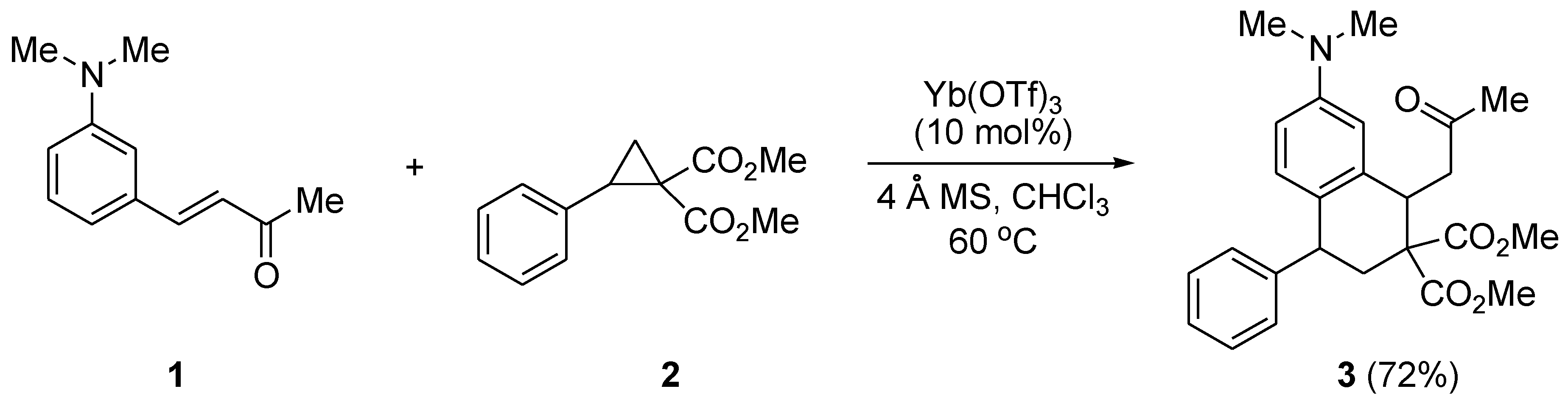
3.1.2. Synthesis of Dimethyl 7-(dimethylamino)-3,4-dihydro-1-(2-oxopropyl)-4-phenylnaphthalene-2,2(1H)-dicarboxylate (3)
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Xu, H.; Lv, M.; Tian, X. A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003–2007. Curr. Med. Chem. 2009, 16, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Iacovino, R.; Izzo, A.; Uzzo, P.; Russo, A.; di Blasio, B.; Monaco, P. Carexanes from Carex distachya Desf.: Revised stereochemistry and characterization of four novel polyhydroxylated prenylstilbenes. Tetrahedron 2008, 64, 7782–7786. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Natale, A.; Monaco, P. Structures of bioactive carexanes from the roots of Carex distachya Desf. Phytochemistry 2006, 67, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.S. Lignans, neolignans and related compounds. Nat. Prod. Rep. 1999, 16, 75–96. [Google Scholar] [CrossRef]
- Imbert, T.F. Discovery of podophyllotoxins. Biochimie 1998, 80, 207–222. [Google Scholar] [CrossRef]
- Damayanthi, Y.; Lown, J.W. Podophyllotoxins: Current status and recent developments. Curr. Med. Chem. 1998, 5, 205–252. [Google Scholar] [PubMed]
- Sun, J.-S.; Liu, H.; Guo, X.-H.; Liao, J.-X. The chemical synthesis of aryltetralin glycosides. Org. Biomol. Chem. 2016, 14, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-Y.; Chen, S.-L.; Yang, M.-H.; Wu, J.; Sinkkonen, J.; Zou, K. An update on lignans: Natural products and synthesis. Nat. Prod. Rep. 2009, 26, 1251–1292. [Google Scholar] [CrossRef] [PubMed]
- Sin, S.; Kim, S.-G. Stereoselective cascade reactions of donor-acceptor cyclopropanes with m-N,N-dialkylaminophenyl α,β-unsaturated carbonyls: Facile diastereoselective synthesis of cis- and trans-tetralins. Adv. Synth. Catal. 2016, 358, 2701–2706. [Google Scholar] [CrossRef]
- Carson, J.R. Aralykyl (arylethynyl)aralkyl Amines and Their Use as Vasodilators and Antihypertensives. U.S. Patent 4661635, 28 April 1987. [Google Scholar]
- Goldberg, A.F. G.; O’Connor, N.R.; Craig, R.A., II; Stoltz, B.M. Lewis acid mediated (3 + 2) cycloaddition of donor-acceptor cyclopropanes with heterocumulenes. Org. Lett. 2012, 14, 5314–5317. [Google Scholar] [CrossRef] [PubMed]
- Cody, J.; Fahrni, C.J. Fluorescence sensing based on cation-induced conformational switching: Copper-selective modulation of the photoinduced intramolecular charge transfer of a donor–acceptor biphenyl fluorophore. Tetrahedron 2004, 60, 11099. [Google Scholar] [CrossRef]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-G. Dimethyl 7-(dimethylamino)-3,4-dihydro-1-(2-oxopropyl)-4-phenylnaphthalene-2,2(1H)-dicarboxylate. Molbank 2017, 2017, M933. https://doi.org/10.3390/M933
Kim S-G. Dimethyl 7-(dimethylamino)-3,4-dihydro-1-(2-oxopropyl)-4-phenylnaphthalene-2,2(1H)-dicarboxylate. Molbank. 2017; 2017(1):M933. https://doi.org/10.3390/M933
Chicago/Turabian StyleKim, Sung-Gon. 2017. "Dimethyl 7-(dimethylamino)-3,4-dihydro-1-(2-oxopropyl)-4-phenylnaphthalene-2,2(1H)-dicarboxylate" Molbank 2017, no. 1: M933. https://doi.org/10.3390/M933




