Introduction
Sensors for blood glucose concentration are of great medical and commercial interest. Most glucose sensors are based upon the amperometric response of electrodes coated with glucose responsive materials; as the glucose concentration changes so does the electrical properties of the responsive materials, and hence the electrical current measured across the electrodes [
1,
2,
3,
4]. A limitation of these sensors is that they require direct wire connections between the electrodes and the detection electronics, which generally precludes their use
in-vivo. In contrast to electronic devices requiring direct connections, magnetism-based devices operate through monitoring changes in magnetic flux using a remotely located pick-up coil. The remote nature of magnetism-based devices offers exciting opportunities for both
in-situ and
in-vivo monitoring.
In this paper we present two wireless, passive magnetism-based glucose sensors that operate in combination with a mass and volume changing glucose responsive polymer. One sensor consists of magnetically soft (low coercive force, high permeability) thin-film elements adhered to a thick film of the glucose responsive polymer. The distance between the magnetic elements changes as the polymer swells and shrinks in response to changes in glucose concentration, changing the magnetostatic coupling between elements. This change in magnetostatic coupling between elements in turn modulates the coercive force and BH loop switching characteristics of the sensor. The other sensor is based on the mass-sensitive change in the magnetoelastic resonant frequency of an amorphous ferromagnetic glass ribbon coated with the glucose responsive polymer. In a manner analogous to mechanical resonators, for small mass loads, increasing mass linearly shift the resonant frequency of the magnetoelastic sensor lower. Both sensors convey information through magnetic flux, hence no electrical wire connections nor specific alignments are required. In addition, both sensors are powered by externally applied magnetic fields, so there are no power lifetime issues such as those associated with battery drain.
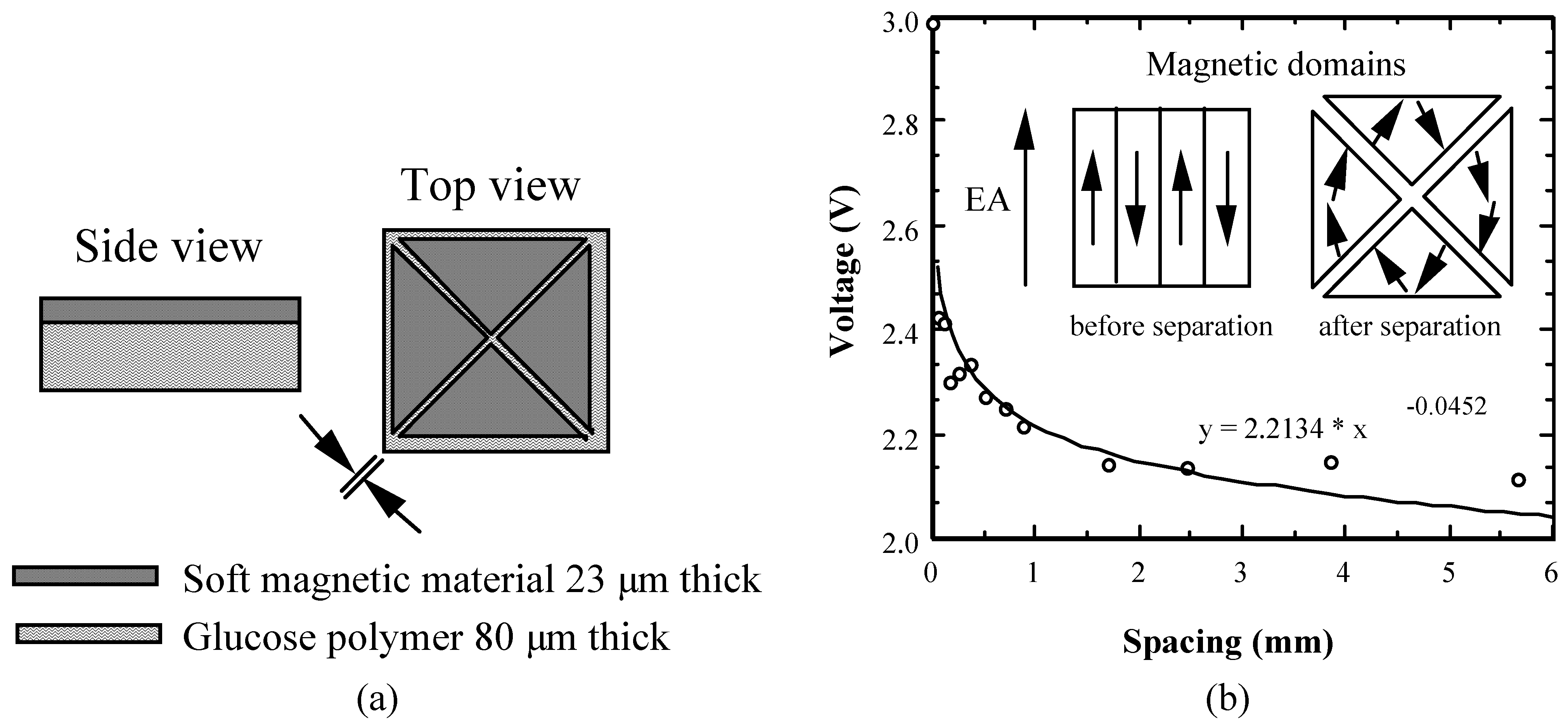
Figure 1.
(a) Schematic drawing of the magnetostatic coupling glucose sensor. Initial spacing between elements is 50 µm, distance expands upon wetting and exposure to glucose. (b) Measured voltage spike amplitude vs. element spacing as a function of measured distance across diagonal. Domain drawing is schematic.
Figure 1.
(a) Schematic drawing of the magnetostatic coupling glucose sensor. Initial spacing between elements is 50 µm, distance expands upon wetting and exposure to glucose. (b) Measured voltage spike amplitude vs. element spacing as a function of measured distance across diagonal. Domain drawing is schematic.
The magnetostatic coupling based sensor is shown in
Fig. 1a. The sensor is composed of four triangular shaped, 23 µm-thick magnetically soft
Atalante® films [
5] bonded onto a physically robust 20 µm thick film of a glucose responsive polymer (to be described) that swells/shrinks in response to changing glucose concentration. The triangles are spaced 50 µm apart and arranged to form a 12 mm × 12 mm square with the triangle tips centered at a common origin. When placed in an ac magnetic field of a magnitude sufficient to saturate the sample, ≈ 0.5 Oe, the time varying flux associated with the magnetization reversals is detected by a nearby pick-up coil as a series of voltage spikes. As the polymer swells, the distance between the triangles increases resulting in a decrease in the magnetostatic coupling, and hence both an increase in coercive force and less coherent switching characteristics, i.e. time rate of change of flux, reducing the amplitude of the voltage spike detected in the pick-up per Faraday’s law (see
Fig. 1b).
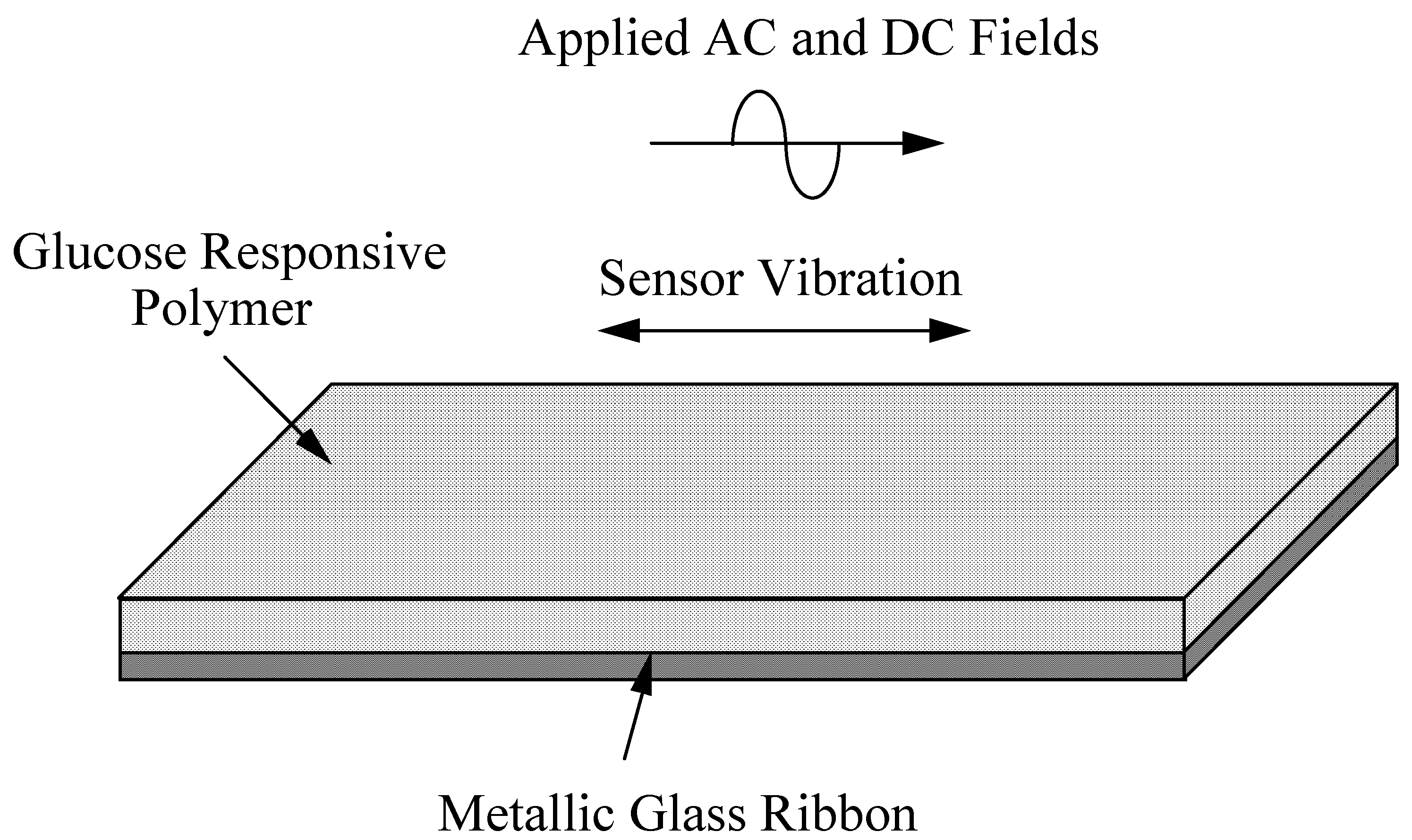
Figure 2.
The magnetoelastic glucose sensor is made of an amorphous metallic glass ribbon coated with a thin layer of mass changing glucose responsive polymer.
Figure 2.
The magnetoelastic glucose sensor is made of an amorphous metallic glass ribbon coated with a thin layer of mass changing glucose responsive polymer.
Another magnetism-based remote query sensor design consists of a magnetoelastic Metglas
TM 2626MB [
6] ribbon that in response to an ac magnetic field demonstrates a characteristic mechanical resonant frequency. The glucose responsive polymer, described below, is adhered to the magnetoelastic ribbon as illustrated in
Fig. 2. As the mass of the polymer changes in response to glucose concentration, so does the resonant frequency of the sensor. The behavior of the magnetoelastic sensor can be approximated by considering the fundamental resonant frequency
f0 of a thin rectangular plate vibrating in its basal plane [
7]:
where
E is Young’s modulus of elasticity,
L is the length of the plate,
ρ is the density, and
σ is the Poisson’s ratio of the sensor. Provided that the mass change is small, the shift in the sensor resonant frequency with applied mass is given by:
where Δ
f is the resonant frequency shift, Δ
m is the change of mass, and
M is the initial mass of the sensor and the polymer layer. The negative sign in Eq. (2) indicates the resonant frequency decreases when the mass of the polymer increases.
The polymer membrane used in this work is made of polyvinyl alcohol (PVA) and a glucose responsive co-polymer the fabrication details of which are given in the next section. In response to varying glucose concentrations the polymer changes size, absorbing corresponding amounts of solution, and thereby changing mass. The time needed for the glucose polymer to reach equilibrium depends on the membrane thickness, glucose concentration, and temperature (all measurements reported on herein were conducted at 23 °C).
Fabrication of Glucose Responsive Polymer
The glucose responsive polymer membrane used in our experiments was made of polyvinyl alcohol (PVA) and a co-polymer consists of N,N-Dimethylacrylamide (DMAA), butyl methacrylate (BMA), N-[3-(dimethylamino) propyl] acrylamide (DMAPAA) and 3-Methacrylamido-phenylboronic acid (MAAPBA).
Preparation of raw materials: DMAA, BMA, DMAPAA, and acrylic acid were purchased from Aldrich Inc. and purified by distillation under reduced pressure before they were used. N,N’-Azobisisobutyronitrile (AIBN) was obtained from Aldrich and purified by recrystallization from ethanol solution at –10 °C. MAAPBA was prepared with the following steps: 100 ml of water and 0.1 mole of 3-aminophenylboronic acid hemisulphate were combined in a 500 ml beaker and stirred with a magnetic stirring bar. The mixture was adjusted to pH 4.8 with 3M NaOH solution and cooled to 4 °C. After that, 0.1 mole of 1-[3(dimethylamino) propyl]3-ethylcarbodiimide hydrochloride and 0.1 mole of acrylic acid were sequentially added while the pH was maintained at 4.8 and the solution temperature 4 °C. After 1 hour of stirring, the mixture was extracted with diethyl ether with a reaction yield of 65%.
Synthesis of glucose sensitive co-polymer: 30 ml of ethanol, 10 mmole of MAAPBA, 10 mmole of DMAA, 10 mmole of BMA and 20 mmole of DMAPAA were combined in a 100 ml flask. The mixture was stirred with a magnetic stirring bar and bubbled with N2 for 20 minutes. This was followed by adding 33 mg of AIBN and then stirring and bubbling with N2 gas for another 30 minutes. The solution was heated to 70~75°C and the reaction was carried out for 3 hours under N2 atmosphere with stirring. The co-polymer was obtained by precipitation from diethyl ether and dried under vacuum condition. The yield was 60-75%.
Formation of glucose-sensitive co-polymer and PVA films: 0.2 wt% glucose sensitive co-polymer solution and 0.2 wt% PVA solution were mixed and the mixture was cast into a model cell. After the solvents evaporated, transparent uniform films were formed. To cross-link the PVA hydroxyl groups that did not complex with the boronic acid moieties in the co-polymer, the films were immersed into 0.177 mmole/L triisocyanate pyridine solution for 10 minutes under N2 atmosphere, then the films were cross-linked in the triisocyanate pyridine solution for 4 hours at 80 °C under N2 atmosphere. The cross-linked films were dried in vacuum for 4 hours.
Glucose sensing mechanism of the polymer: When glucose complexes with the hydroxyl groups of the boronate moieties, it is inserted between the two polymer chains, increasing the distance between the chains of the co-polymer and PVA. This in turn increases the capacity of the membrane to absorb water, thereby increasing in volume and mass. When the ratio of MAAPBA in the co-polymers is increased the glucose responding PVA-membranes become harder and there is less polymer swelling. When the ratio of DMAPAA in the co-polymers is increased the glucose responding membranes become softer with greater swelling. It is believed that with fewer phenylboronic acid moieties, the intermolecular chains between the two polymers are weaker, so more water molecules can be absorbed, causing a higher change in volume and mass.
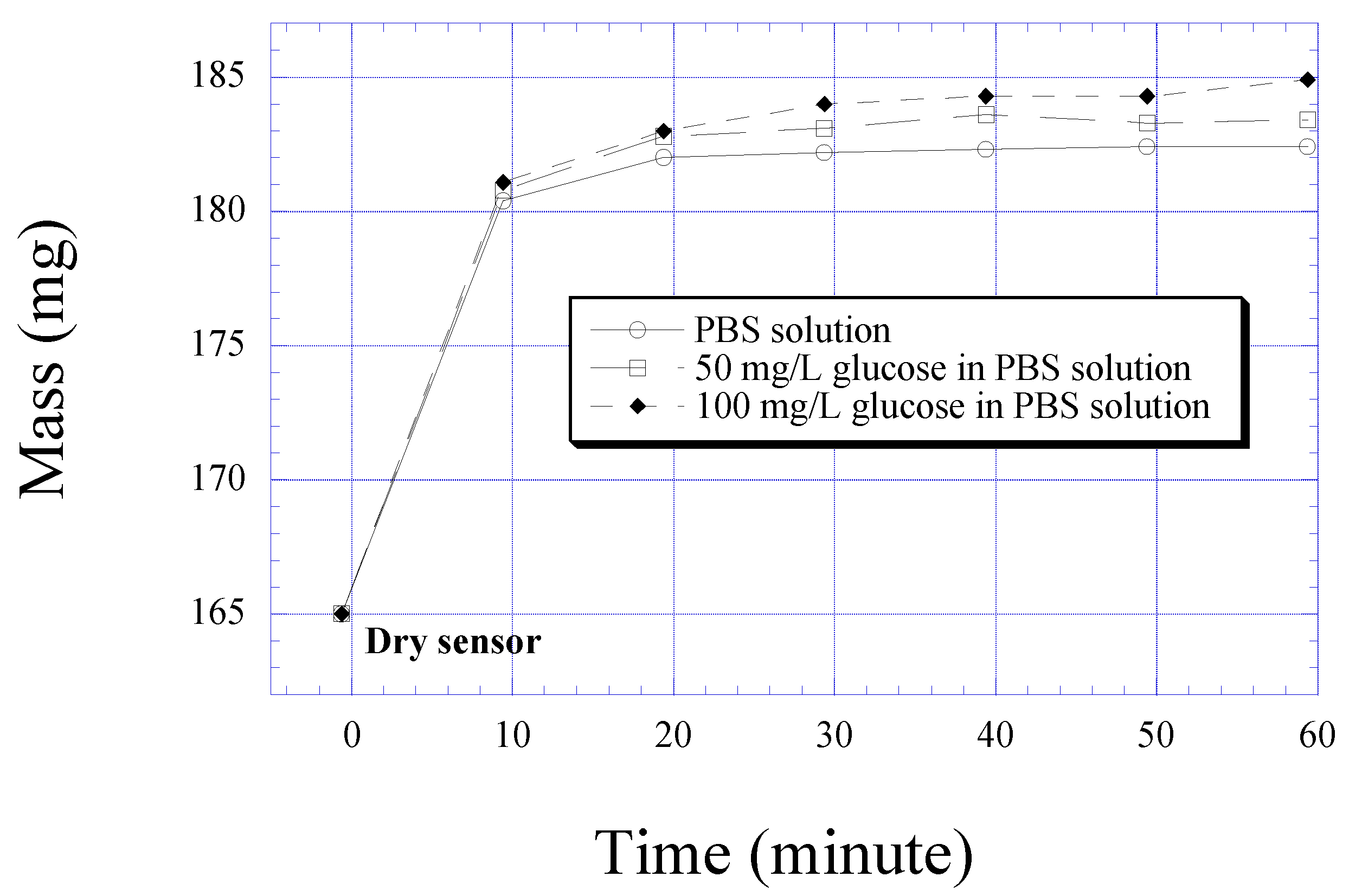
Figure 3.
Mass change of a 12 µm thick glucose responding polymer layer as it is immersed into phosphate buffered saline solution with and without glucose.
Figure 3.
Mass change of a 12 µm thick glucose responding polymer layer as it is immersed into phosphate buffered saline solution with and without glucose.
Fig. 3 demonstrates the change in the polymer mass as it changes from a dry to wet polymer, and as a function of glucose concentration. As seen, the largest change in mass is due to the polymer simply changing from dry to wet, however there is still a useful differential change in mass with glucose concentration. Measuring volume change is much more difficult, as the polymer softens upon immersion in solution; it was generally estimated that the volume changed approximately 5% from dry to wet.
Conclusions
In combination with a mass and volume changing glucose responsive polymer, the design and application of two magnetism-based glucose sensors are presented. The magnetostatic coupling glucose sensor consists of four triangularly shaped magnetically soft thin-film elements attached to a volume-changing glucose responsive thick film polymer. As seen in
Figure 3, in response to increasing glucose concentration the polymer swells, which also serves to increase its mass. As the polymer swells, the distance between the magnetic elements increases. This increased spacing between elements in turn results in less magnetostatic coupling, so that when the magnetization vectors of each element rotate they do so individually. The non-coherent rotation of the magnetization vectors results in a smaller time rate of change of flux and hence, as per Faraday’s law, a smaller generated voltage spike amplitude in the monitoring pick-up coil. With reference to
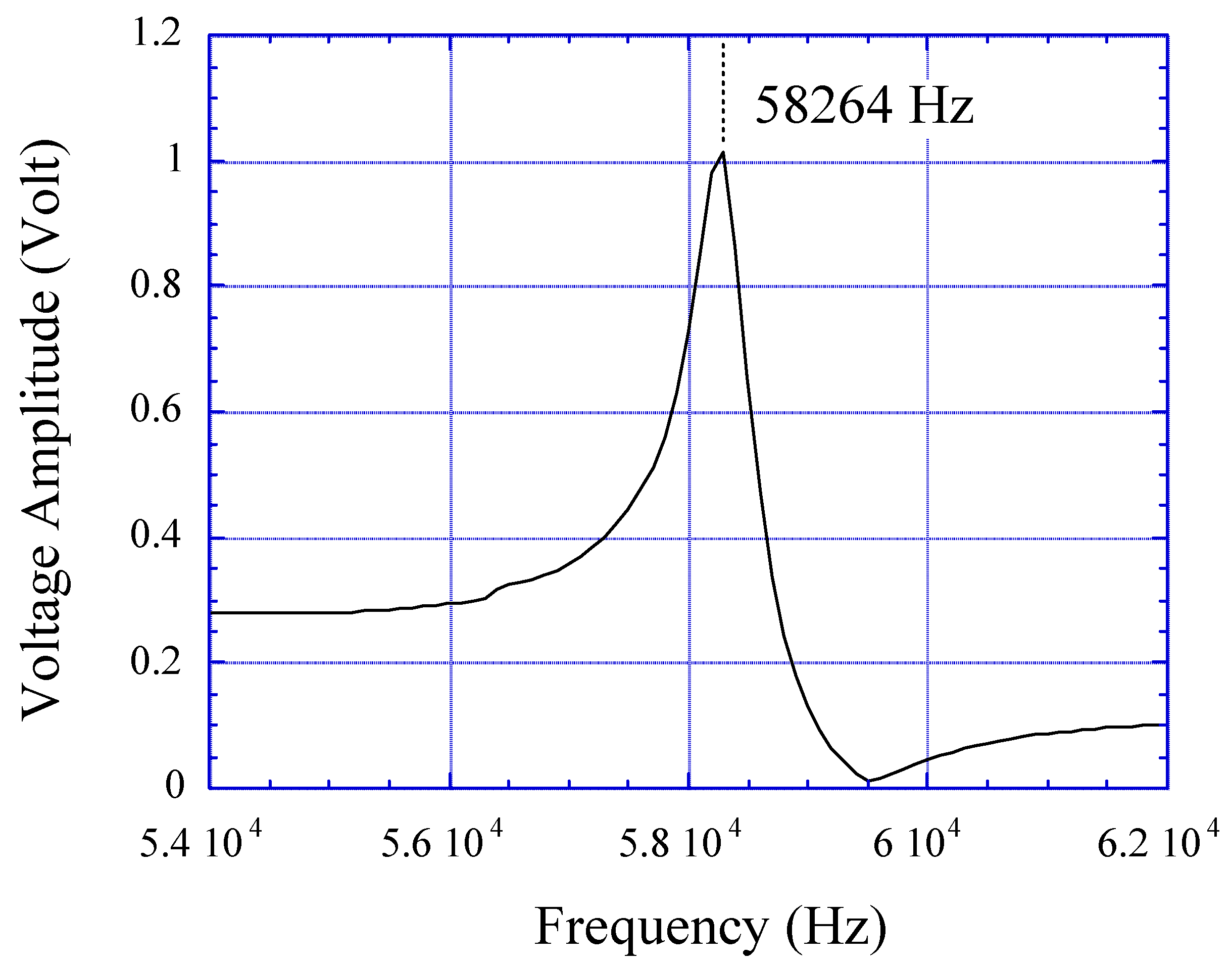
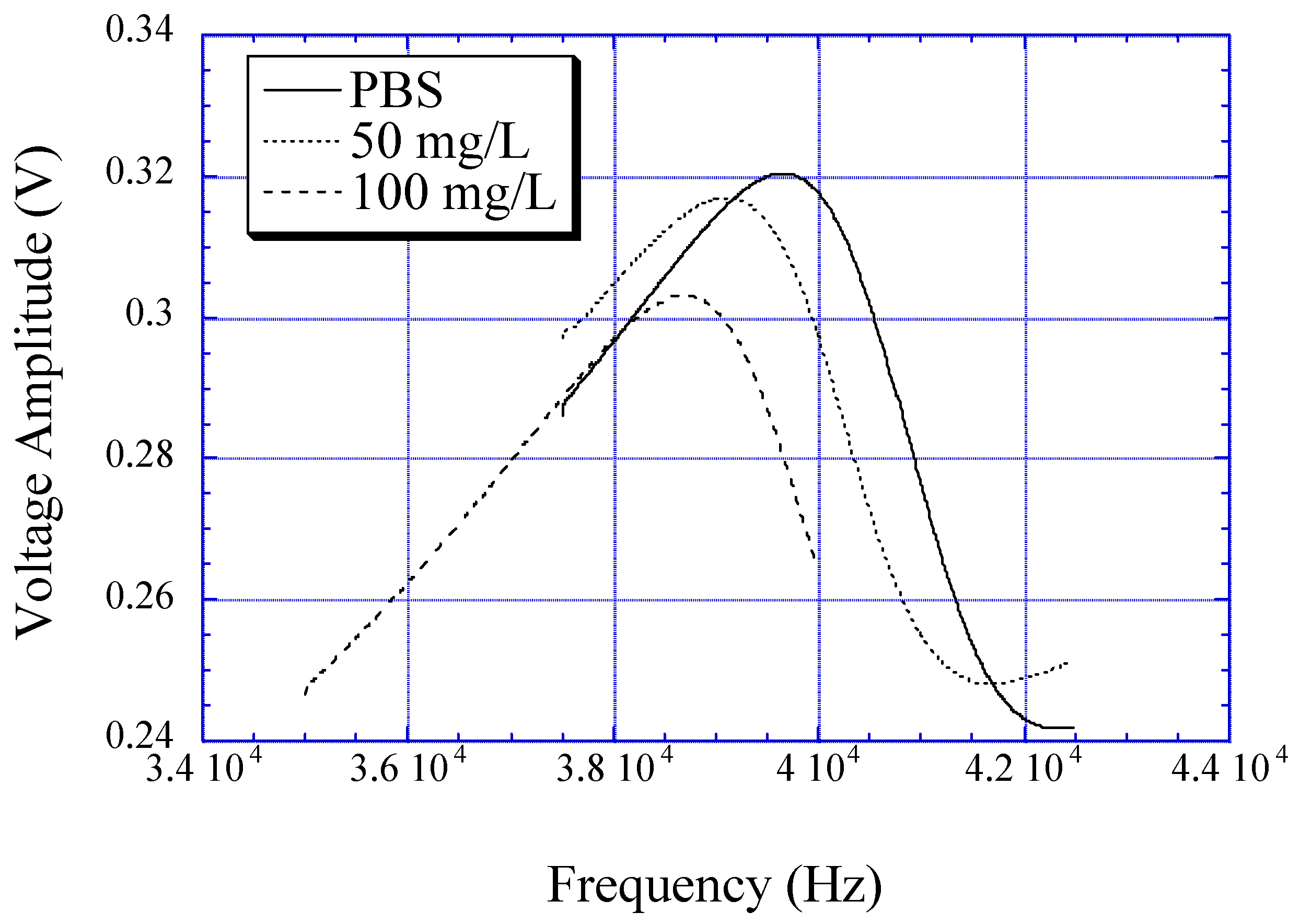
Figure 4, the initial upward trend in the amplitude of the measured voltage spike is due to curling of the sensor that brings the magnetic elements closer. After equilibrium is achieved, the glucose solution demonstrates a decrease in the detected voltage spike amplitude due to less magnetostatic coupling between elements.
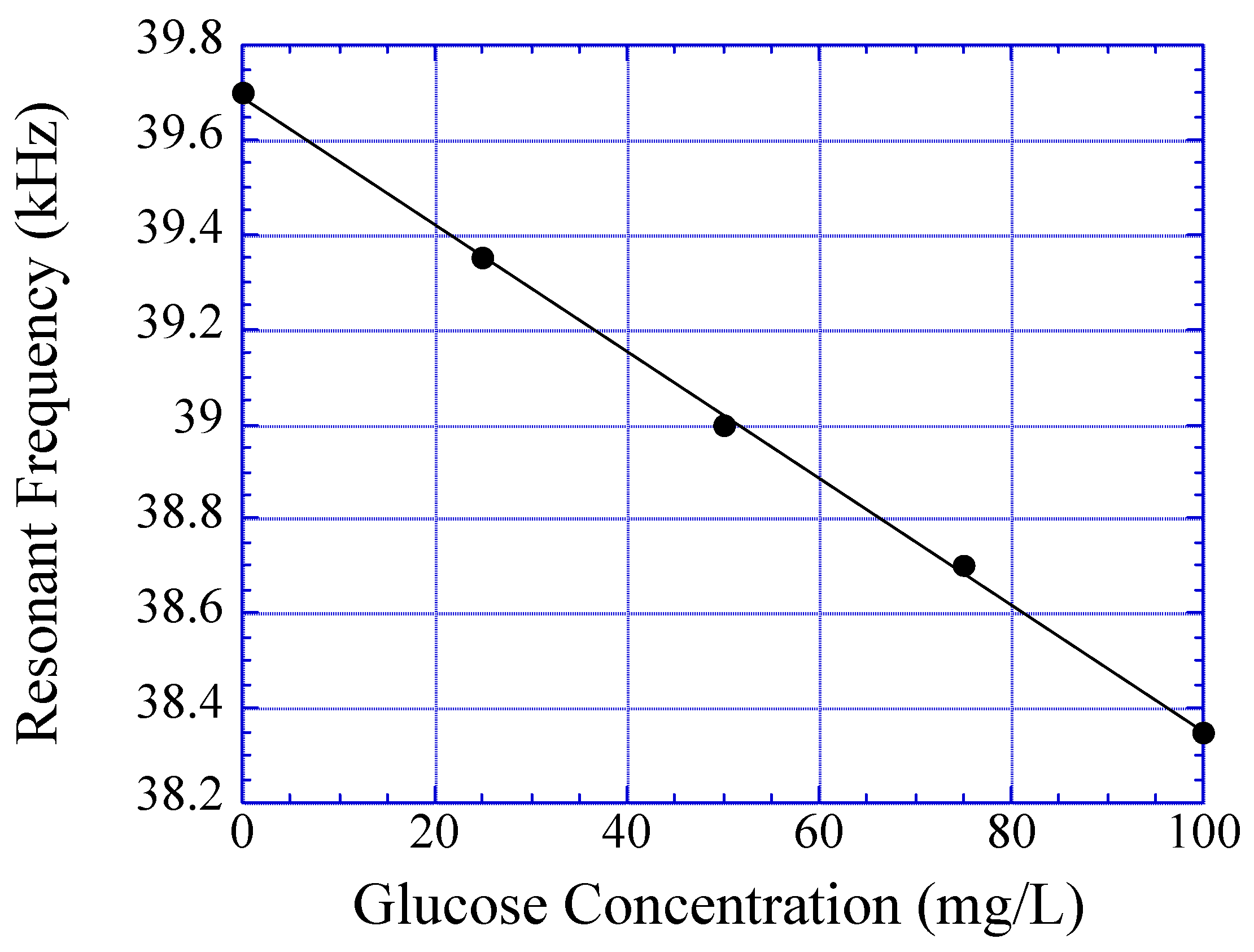
Experimental results show the resonant frequency of the magnetoelastic glucose sensor linearly decreases with increasing glucose concentration due to the effect of increasing polymer mass. Although the mass of the polymer changes with glucose concentration, the saturation time for the analyte to diffuse into the homogeneous polymer layer is too long for most useful applications. Using polymer strips or beads rather than thick films could enhance the response time of the polymer.
The remote query nature of the magnetism based glucose sensors offers the possibility that, with the sensors coated with a suitable biocompatible coating such as PEG, the sensors could be placed inside a patient and monitored remotely without the need for blood sampling or direct connections. Since the sensors are passive, with no internal power supplies, they could last indefinitely as dependent upon the stability of the glucose responding polymer.