Optical Oxygen Sensors for Applications in Microfluidic Cell Culture
Abstract
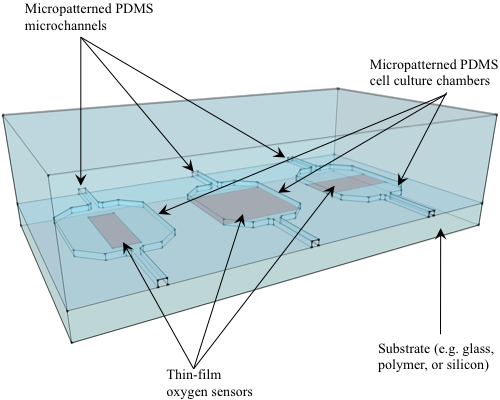
:1. Introduction
1.1. Oxygen and Cells
1.2. Microfluidics for Cell Culture and Cell-Based Studies
1.3. Oxygen Sensors
2. Optical Oxygen Sensing Methods
2.1. Detection of Luminescence Intensity
2.2. Detection of Luminescence Lifetime
3. Oxygen-Sensitive Luminescent Materials
3.1. Ruthenium-based
3.2. Metalloporphyrin-based
3.3. Summary and Applicability to Microfluidic Cell Culture
4. Indicator Encapsulation Media
4.1. Polymers
4.2. Silica, Ormosil, and Sol-gel
5. Oxygen Sensor Formats
5.1. Thin-film Sensors on Substrate
5.2. Optical Fiber Sensors
5.3. PEBBLE/Microparticle/Nanoparticle Sensors
5.4. Water-Soluble/Macromolecular Probes
6. Optical Measurement Systems
6.1. Excitation Light Sources
6.2. Detectors
6.3. Imaging Systems
7. Optical Oxygen Sensors in Microfluidic Cell Culture and Analysis
8. Conclusions
Acknowledgments
References and Notes
- Bunn, HF; Poyton, RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev 1996, 76, 839–885. [Google Scholar]
- Ivanovic, Z. Hypoxia or in situ normoxia: The stem cell paradigm. J. Cell Physiol 2009, 219, 271–275. [Google Scholar]
- Goligorsky, MS. Making sense out of oxygen sensor. Circ. Res 2000, 86, 824–826. [Google Scholar]
- Lahiri, S. Historical perspectives of cellular oxygen sensing and responses to hypoxia. J. Appl. Physiol 2000, 88, 1467–1473. [Google Scholar]
- Hockel, M; Vaupel, P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Nat. Cancer Inst 2001, 93, 266–276. [Google Scholar]
- Harris, AL. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar]
- Arbeit, JM; Brown, JM; Chao, KSC; Chapman, JD; Eckelman, WC; Fyles, AW; Giaccia, AJ; Hill, RP; Koch, CJ; Krishna, MC; Krohn, KA; Lewis, JS; Mason, RP; Melillo, G; Padhani, AR; Powis, G; Rajendran, JG; Reba, R; Robinson, SP; Semenza, GL; Swartz, HM; Vaupel, P; Yang, D. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int. J. Radiat. Biol 2006, 82, 699–757. [Google Scholar]
- Csete, M. Oxygen in the cultivation of stem cells. Ann. N.Y. Acad. Sci 2005, 1049, 1–8. [Google Scholar]
- Simon, MC; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Bio 2008, 9, 285–296. [Google Scholar]
- Panchision, DM. The role of oxygen in regulating neural stem cells in development and disease. J. Cell Physiol 2009, 220, 562–568. [Google Scholar]
- Papkovsky, DB; Ovchinnikov, AN; Ogurtsov, VI; Ponomarev, GV; Korpela, T. Biosensors on the basis of luminescent oxygen sensor: The use of microporous light-scattering support materials. Sens. Actuat. B Chem 1998, 51, 137–145. [Google Scholar]
- Papkovsky, DB; O' Riordan, T; Soini, A. Phosphorescent porphyrin probes in biosensors and sensitive bioassays. Biochem. Soc. Trans 2000, 28, 74–77. [Google Scholar]
- Brown, JQ; Srivastava, R; McShane, MJ. Encapsulation of glucose oxidase and an oxygen-quenched fluorophore in polyelectrolyte-coated calcium alginate microspheres as optical glucose sensor systems. Biosens. Bioelectron 2005, 21, 212–216. [Google Scholar]
- Yotter, RA; Lee, LA; Wilson, DM. Sensor technologies for monitoring metabolic activity in single cells - Part I: Optical, methods. IEEE Sensors J 2004, 4, 395–411. [Google Scholar]
- Beebe, DJ; Mensing, GA; Walker, GM. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng 2002, 4, 261–286. [Google Scholar]
- Whitesides, GM. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar]
- Becker, H; Gartner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem 2008, 390, 89–111. [Google Scholar]
- Dittrich, PS; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov 2006, 5, 210–218. [Google Scholar]
- Andersson, H; van den Berg, A. Microfluidic devices for cellomics: A review. Sens. Actuat B-Chem 2003, 92, 315–325. [Google Scholar]
- Yi, CQ; Li, CW; Ji, SL; Yang, MS. Microfluidics technology for manipulation and analysis of biological cells. Anal.Chim. Acta 2006, 560, 1–23. [Google Scholar]
- El-Ali, J; Sorger, PK; Jensen, KF. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar]
- Keenan, TM; Folch, A. Biomolecular gradients in cell culture systems. Lab Chip 2008, 8, 34–57. [Google Scholar]
- Meyvantsson, I; Beebe, DJ. Cell culture models in microfluidic systems. Ann. Rev. Anal. Chem 2008, 1, 423–449. [Google Scholar]
- Salieb-Beugelaar, GB; Simone, G; Arora, A; Philippi, A; Manz, A. Latest developments in microfluidic cell biology and analysis systems. Anal. Chem 2010, 82, 4848–4864. [Google Scholar]
- Erickson, D; Li, DQ. Integrated microfluidic devices. Anal.Chim. Acta 2004, 507, 11–26. [Google Scholar]
- Abgrall, P; Gue, AM. Lab-on-chip technologies: Making a microfluidic network and coupling it into a complete microsystem—A review. J. Microme. Microen 2007, 17, R15–R49. [Google Scholar]
- Puccinelli, JP; Su, X; Beebe, DJ. Automated high-throughput microchannel assays for cell biology: Operational optimization and characterization. J. Assoc. Lab. Autom 2010, 15, 25–32. [Google Scholar]
- Liebsch, G; Klimant, I; Frank, B; Holst, G; Wolfbeis, OS. Luminescence lifetime imaging of oxygen, pH, and carbon dioxide distribution using optical sensors. Appl. Spectrosc 2000, 54, 548–559. [Google Scholar]
- Kocincova, AS; Nagl, S; Arain, S; Krause, C; Borisov, SM; Arnold, M; Wolfbeis, OS. Multiplex bacterial growth monitoring in 24-well microplates using a dual optical sensor for dissolved oxygen and pH. Biotechnol. Bioeng 2008, 100, 430–438. [Google Scholar]
- Liebsch, G; Klimant, I; Krause, C; Wolfbeis, OS. Fluorescent imaging of pH with optical sensors using time domain dual lifetime referencing. Anal. Chem 2001, 73, 4354–4363. [Google Scholar]
- Huang, CW; Lee, GB. A microfluidic system for automatic cell culture. J. Microme. Microen 2007, 17, 1266–1274. [Google Scholar]
- Lao, AIK; Lee, TMH; Hsing, IM; Ip, NY. Precise temperature control of microfluidic chamber for gas and liquid phase reactions. Sens. Actuat. A-Phys 2000, 84, 11–17. [Google Scholar]
- Clark, LC; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Acad. Sci 1962, 102, 29–45. [Google Scholar]
- Wu, CC; Yasukawa, T; Shiku, H; Matsue, T. Fabrication of miniature Clark oxygen sensor integrated with microstructure. Sens. Actuat. B-Chem 2005, 110, 342–349. [Google Scholar]
- Suresh, S; Srivastava, VC; Mishra, IM. Techniques for oxygen transfer measurement in bioreactors: a review. J. Chem. Technol. Biotechnol 2009, 84, 1091–1103. [Google Scholar]
- Gatti, S; Brey, T; Muller, WEG; Heilmayer, O; Holst, G. Oxygen microoptodes: a new tool for oxygen measurements in aquatic animal ecology. Marine Biol 2002, 140, 1075–1085. [Google Scholar]
- Mills, A. Optical oxygen sensors. Platinum Met. Rev 1997, 41, 115–127. [Google Scholar]
- Hartmann, P; Ziegler, W; Holst, G; Lubbers, DW. Oxygen flux fluorescence lifetime imaging. Sens. Actuat B-Chem 1997, 38, 110–115. [Google Scholar]
- Park, EJ; Reid, KR; Tang, W; Kennedy, RT; Kopelman, R. Ratiometric fiber optic sensors for the detection of inter- and intra-cellular dissolved oxygen. J. Mater. Chem 2005, 15, 2913–2919. [Google Scholar]
- Stern, O; Volmer, M. The fading time of fluorescence. Phys. Z 1919, 20, 183–188. [Google Scholar]
- Konig, B; Kohls, O; Holst, G; Glud, RN; Kuhl, M. Fabrication and test of sol-gel based planar oxygen optodes for use in aquatic sediments. Mar Chem 2005, 97, 262–276. [Google Scholar]
- Bergman, I. Rapid-response atmospheric oxygen monitor based on fluorescence quenching. Nature 1968, 218, 396. [Google Scholar]
- Wilson, DF; Vinogradov, SA. Tissue oxygen measurements using phosphorescence quenching. In Handbook of Biomedical Fluorescence; Mycek, MA, Pogue, BW, Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 637–662. [Google Scholar]
- Amao, Y. Probes and polymers for optical sensing of oxygen. Microchim. Acta 2003, 143, 1–12. [Google Scholar]
- McDonagh, C; Burke, CS; MacCraith, BD. Optical chemical sensors. Chem. Rev 2008, 108, 400–422. [Google Scholar]
- Holst, G; Kohls, O; Klimant, I; Konig, B; Kuhl, M; Richter, T. A modular luminescence lifetime imaging system for mapping oxygen distribution in biological samples. Sens. Actuat. B-Chem 1998, 51, 163–170. [Google Scholar]
- Itoh, T; Yaegashi, K; Kosaka, T; Kinoshita, T; Morimoto, T. In-Vivo visualization of oxygen-transport in microvascular network. Am. J. Physiol-Heart C 1994, 36, H2068–H2078. [Google Scholar]
- Nock, V; Blaikie, RJ; David, T. Patterning, integration and characterisation of polymer optical oxygen sensors for microfluidic devices. Lab Chip 2008, 8, 1300–1307. [Google Scholar]
- Rumsey, WL; Vanderkooi, JM; Wilson, DF. Imaging of phosphorescence—A novel method for measuring oxygen distribution in perfused tissue. Science 1988, 241, 1649–1651. [Google Scholar]
- Chang-Yen, DA; Badardeen, A; Gale, BK. Spin-assembled nanofilms for gaseous oxygen sensing. Sens. Actuat. B-Chem 2007, 120, 426–433. [Google Scholar]
- Koo, Y-EL; Cao, Y; Kopelman, R; Koo, SM; Brasuel, M; Philbert, MA. Real-time measurements of dissolved oxygen inside live cells by organically modified silicate fluorescent nanosensors. Anal. Chem 2004, 76, 2498–2505. [Google Scholar]
- O’Donovan, C; Hynes, J; Yashunski, D; Papkovsky, DB. Phosphorescent oxygen-sensitive materials for biological applications. J. Mater. Chem 2005, 15, 2946–2951. [Google Scholar]
- Lin, Z; Cherng-Wen, T; Roy, P; Trau, D. In-situ measurement of cellular microenvironments in a microfluidic device. Lab Chip 2009, 9, 257–262. [Google Scholar]
- Lam, RHW; Kim, M-C; Thorsen, T. A microfluidic oxygenator for biological cell culture. Proceedings of The 14th International Conference on Solid-State Sensors, Actuators, and Microsystems, Transducers & Eurosensors '07, Lyon, France, June 10–14, 2007.
- Lam, RH; Kim, MC; Thorsen, T. Culturing aerobic and anaerobic bacteria and mammalian cells with a microfluidic differential oxygenator. Anal. Chem 2009, 81, 5918–5924. [Google Scholar]
- Cao, Y; Lee Koo, YE; Kopelman, R. Poly(decyl methacrylate)-based fluorescent PEBBLE swarm nanosensors for measuring dissolved oxygen in biosamples. Analyst 2004, 129, 745–750. [Google Scholar]
- Borisov, SM; Klimant, I. Luminescent nanobeads for optical sensing and imaging of dissolved oxygen. Microchim. Acta 2009, 164, 7–15. [Google Scholar]
- Elder, AD; Matthews, SM; Swartling, J; Yunus, K; Frank, JH; Brennan, CM; Fisher, AC; Kaminski, CF. The application of frequency-domain Fluorescence Lifetime Imaging Microscopy as a quantitative analytical tool for microfluidic devices. Opt. Express 2006, 14, 5456–5467. [Google Scholar]
- Holst, G; Glud, RN; Kuhl, M; Klimant, I. A microoptode array for fine-scale measurement of oxygen distribution. Sens. Actuat. B-Chem 1997, 38, 122–129. [Google Scholar]
- Klimant, I; Kuhl, M; Glud, RN; Holst, G. Optical measurement of oxygen and temperature in microscale: strategies and biological applications. Sens. Actuat. B-Chem 1997, 38, 29–37. [Google Scholar]
- Papkovsky, DB; Ponomarev, GV; Trettnak, W; O' Leary, P. Phosphorescent complexes of porphyrin ketones: Optical properties and application to oxygen sensing. Anal. Chem 1995, 67, 4112–4117. [Google Scholar]
- Kellner, K; Liebsch, G; Klimant, I; Wolfbeis, OS; Blunk, T; Schulz, MB; G’ pferich, A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol. Bioeng 2002, 80, 73–83. [Google Scholar]
- Holst, G; Grunwald, BR. Luminescence lifetime imaging with transparent oxygen optodes. Sens. Actuat. B: Chem 2001, 74, 78–90. [Google Scholar]
- Urayama, P; Zhong, W; Beamish, JA; Minn, FK; Sloboda, RD; Dragnev, KH; Dmitrovsky, E; Mycek, MA. A UV-visible-NIR fluorescence lifetime imaging microscope for laser-based biological sensing with picosecond resolution. Appl. Phys. B-Lasers O 2003, 76, 483–496. [Google Scholar]
- Wang, XF; Uchida, T; Coleman, DM; Minami, S. A 2-dimensional fluorescence lifetime imaging-system using a gated image intensifier. Appl. Spectrosc 1991, 45, 360–366. [Google Scholar]
- Zhong, W; Urayama, P; Mycek, MA. Imaging fluorescence lifetime modulation of a ruthenium-based dye in living cells: the potential for oxygen sensing. J. Phys. D Appl. Phys 2003, 36, 1689–1695. [Google Scholar]
- Marriott, G; Clegg, RM; Arndtjovin, DJ; Jovin, TM. Time resolved imaging microscopy—phosphorescence and delayed fluorescence imaging. Biophys. J 1991, 60, 1374–1387. [Google Scholar]
- Benninger, RKP; Hofmann, O; McGinty, J; Requejo-Isidro, J; Munro, I; Neil, MAA; deMello, AJ; French, PMW. Time-resolved fluorescence imaging of solvent interactions in microfluidic devices. Opt. Express 2005, 13, 6275–6285. [Google Scholar]
- Shonat, RD; Wilson, DF; Riva, CE; Pawlowski, M. Oxygen distribution in the retinal and choroidal vessels of the cat as measured by a new phosphorescence imaging method. Appl. Opti 1992, 31, 3711–3718. [Google Scholar]
- Sud, D; Zhong, W; Beer, DG; Mycek, MA. Time-resolved optical imaging provides a molecular snapshot of altered metabolic function in living human cancer cell models. Opt. Express 2006, 14, 4412–4426. [Google Scholar]
- Mehta, G; Mehta, K; Sud, D; Song, JW; Bersano-Begey, T; Futai, N; Heo, YS; Mycek, MA; Linderman, JJ; Takayama, S. Quantitative measurement and control of oxygen levels in microfluidic poly(dimethylsiloxane) bioreactors during cell culture. Biomed. Microdevices 2007, 9, 123–134. [Google Scholar]
- Kuhl, M; Rickelt, LF; Thar, R. Combined imaging of bacteria and oxygen in biofilms. Appl. Environ. Microb 2007, 73, 6289–6295. [Google Scholar]
- Lakowicz, JR; Berndt, KW. Lifetime-selective fluorescence imaging using an Rf phase-sensitive camera. Rev. Sci. Instrum 1991, 62, 1727–1734. [Google Scholar]
- Lippitsch, ME; Pusterhofer, J; Leiner, MJP; Wolfbeis, OS. Fibre-optic oxygen sensor with the fluorescence decay time as the information carrier. Anal. Chim. Acta 1988, 205, 1–6. [Google Scholar]
- Berndt, KW; Lakowicz, JR. Electroluminescent lamp-based phase fluorometer and oxygen sensor. Anal. Biochem 1992, 201, 319–325. [Google Scholar]
- Trettnak, W; Kolle, C; Reininger, F; Dolezal, C; OLeary, P. Miniaturized luminescence lifetime-based oxygen sensor instrumentation utilizing a phase modulation technique. Sens. Actuat. B-Chem 1996, 36, 506–512. [Google Scholar]
- Yao, L; Khan, R; Chodavarapu, VP; Tripathi, VS; Bright, FV. Sensitivity-Enhanced CMOS Phase Luminometry System Using Xerogel-Based Sensors. IEEE Trans. Biomed. Circuit. Syst 2009, 3, 304–311. [Google Scholar]
- Vollmer, AP; Probstein, RF; Gilbert, R; Thorsen, T. Development of an integrated microfluidic platform for dynamic oxygen sensing and delivery in a flowing medium. Lab Chip 2005, 5, 1059–1066. [Google Scholar]
- Szita, N; Boccazzi, P; Zhang, ZY; Boyle, P; Sinskey, AJ; Jensen, KF. Development of a multiplexed microbioreactor system for high-throughput bioprocessing. Lab Chip 2005, 5, 819–826. [Google Scholar]
- Zanzotto, A; Szita, N; Boccazzi, P; Lessard, P; Sinskey, AJ; Jensen, KF. Membrane-aerated microbioreactor for high-throughput bioprocessing. Biotechnol. Bioeng 2004, 87, 243–254. [Google Scholar]
- Sin, A; Chin, KC; Jamil, MF; Kostov, Y; Rao, G; Shuler, ML. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotech. Pro 2004, 20, 338–345. [Google Scholar]
- Aubin, JE. Autofluorescence of viable cultured mammalian-cells. J. Histochem. Cytochem 1979, 27, 36–43. [Google Scholar]
- Vanderkooi, JM; Maniara, G; Green, TJ; Wilson, DF. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J. Biol. Chem 1987, 262, 5476–5482. [Google Scholar]
- Papkovsky, DB; O’riordan, TC. Emerging applications of phosphorescent metalloporphyrins. J. Fluoresc 2005, 15, 569–584. [Google Scholar]
- Bacon, JR; Demas, JN. Determination of oxygen concentrations by luminescence quenching of a polymer-immobilized transition-metal complex. Anal. Chem 1987, 59, 2780–2785. [Google Scholar]
- Klimant, I; Meyer, V; Kuhl, M. Fiber-optic oxygen microsensors, a new tool in aquatic biology. Limnol Oceanogr 1995, 40, 1159–1165. [Google Scholar]
- Klimant, I; Ruckruh, F; Liebsch, G; Stangelmayer, C; Wolfbeis, OS. Fast response oxygen micro-optodes based on novel soluble ormosil glasses. Mikrochim. Acta 1999, 131, 35–46. [Google Scholar]
- McDonagh, C; Kolle, C; McEvoy, AK; Dowling, DL; Cafolla, AA; Cullen, SJ; MacCraith, BD. Phase fluorometric dissolved oxygen sensor. Sens. Actuat B-Chem 2001, 74, 124–130. [Google Scholar]
- Lu, X; Han, B-H; Winnik, MA. Characterizing the quenching process for phosphorescent dyes in poly[((n-butylamino)thionyl)phosphazene] films. J. Phys. Chem. B 2003, 107, 13349–13356. [Google Scholar]
- O’Riordan, TC; Voraberger, H; Kerry, JP; Papkovsky, DB. Study of migration of active components of phosphorescent oxygen sensors for food packaging applications. Anal. Chim. Acta 2005, 530, 135–141. [Google Scholar]
- Babilas, P; Liebsch, G; Schacht, V; Klimant, I; Wolfbeis, OS; Szeimies, RM; Abels, C. In vivo phosphorescence imaging of pO2 using planar oxygen sensors. Microcirculation 2005, 12, 477–487. [Google Scholar]
- Klimant, I; Wolfbeis, OS. Oxygen-sensitive luminescent materials based on silicone-soluble ruthenium diimine complexes. Anal. Chem 1995, 67, 3160–3166. [Google Scholar]
- Ambekar, R; Park, J; Henthorn, DB; Kim, C-S. Photopatternable polymeric membranes for optical oxygen sensors. IEEE Sensors J 2009, 9, 169–175. [Google Scholar]
- Chang-Yen, DA; Gale, BK. An integrated optical oxygen sensor fabricated using rapid-prototyping techniques. Lab Chip 2003, 3, 297–301. [Google Scholar]
- Sud, D; Mehta, G; Mehta, K; Linderman, J; Takayama, S; Mycek, M-A. Optical imaging in microfluidic bioreactors enables oxygen monitoring for continuous cell culture. J. Biomed. Opt 2006, 11, 0505041–0505043. [Google Scholar]
- Mongey, K; Vos, JG; Maccraith, BD; Mcdonagh, CM. The photophysical properties of ruthenium polypyridyl complexes within a sol-gel matrix. J. Sol-Gel. Sci. Techn 1997, 8, 979–983. [Google Scholar]
- Eastwood, D; Gouterma, M. Porphyrins : XVIII. Luminescence of (Co), (Ni), Pd, Pt complexes. J. Mol. Spectrosc 1970, 35, 359–375. [Google Scholar]
- Papkovsky, DB; Olah, J; Troyanovsky, IV; Sadovsky, NA; Rumyantseva, VD; Mironov, AF; Yaropolov, AI; Savitsky, AP. Phosphorescent polymer-films for optical oxygen sensors. Biosens. Bioelectron 1992, 7, 199–206. [Google Scholar]
- Papkovsky, DB; Ponomarev, GV; Wolfbeis, OS. Longwave luminescent porphyrin probes. Spectrochim. Acta A 1996, 52, 1629–1638. [Google Scholar]
- Nock, V; Blaikie, RJ; David, T. Micro-patterning of polymer-based optical oxygen sensors for lab-on-chip applications. In Proceedings of the Society of Photo-Optical Instrumentation Engineers (SPIE), BIOMEMS and Nanotechnology III; Nicolau, DV, Abbott, D, KalantarZadeh, K, DiMatteo, T, Bezrukov, SM, Eds.; SPIE: Australian Natl Univ; COSNet ARC; CUDOS; RPO; Bandwidth Foundry; SPIE-Int. Soc.; Optical Engineering: Canberra, ACT, Australia, 2008; pp. 1–10. [Google Scholar]
- Wilson, DF; Rumsey, WL; Green, TJ; Vanderkooi, JM. The oxygen dependence of mitochondrial oxidative-phosphorylation measured by a new optical method for measuring oxygen concentration. J. Biol. Chem 1988, 263, 2712–2718. [Google Scholar]
- Torres Filho, IP; Intaglietta, M. Microvessel Po2 Measurements by phosphorescence decay method. Am. J. Physiol 1993, 265, H1434–H1438. [Google Scholar]
- Wilson, DF; Cerniglia, GJ. Localization of tumors and evaluation of their state of oxygenation by phosphorescence imaging. Cancer Res 1992, 52, 3988–3993. [Google Scholar]
- Cerniglia, GJ; Wilson, DF; Pawlowski, M; Vinogradov, S; Biaglow, J. Intravascular oxygen distribution in subcutaneous 9L tumors and radiation sensitivity. J. Appl. Physiol 1997, 82, 1939–1945. [Google Scholar]
- Smith, LM; Golub, AS; Pittman, RN. Interstitial PO(2) determination by phosphorescence quenching microscopy. Microcirculation 2002, 9, 389–395. [Google Scholar]
- Lo, LW; Koch, CJ; Wilson, DF. Calibration of oxygen-dependent quenching of the phosphorescence of Pd-meso-tetra (4-carboxyphenyl) porphine: A phosphor with general application for measuring oxygen concentration in biological systems. Anal. Biochem 1996, 236, 153–160. [Google Scholar]
- Apreleva, SV; Wilson, DF; Vinogradov, SA. Tomographic imaging of oxygen by phosphorescence lifetime. Appl. Opt 2006, 45, 8547–8559. [Google Scholar]
- Wilson, DF; Vinogadov, SA; Grosul, P; Sund, N; Vacarezza, MN; Bennett, J. Imaging oxygen pressure in the rodent retina by phosphorescence lifetime. Adv. Exp. Med. Biol 2006, 578, 119–124. [Google Scholar]
- Dunphy, I; Vinogradov, SA; Wilson, DF. Oxyphor R2 and G2: Phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence. Anal. Biochem 2002, 310, 191–198. [Google Scholar]
- Johannes, T; Mik, EG; Ince, C. Dual-wavelength phosphorimetry for determination of cortical and subcortical microvascular oxygenation in rat kidney. J. Appl. Physiol 2006, 100, 1301–1310. [Google Scholar]
- Johannes, T; Mik, EG; Ince, C. Nonresuscitated Endotoxemia Induces microcirculatory hypoxic areas in the renal cortex in the rat. Shock 2009, 31, 97–103. [Google Scholar]
- Mik, EG; Johannes, T; Ince, C. Monitoring of renal venous Po-2 and kidney oxygen consumption in rats by a near-infrared phosphorescence lifetime technique. Am. J. Phy.-Ren 2008, 294, F676–F681. [Google Scholar]
- Orth, TA; Allen, JA; Wood, JG; Gonzalez, NC. Exercise training prevents the inflammatory response to hypoxia in cremaster venules. J. Appl. Physiol 2005, 98, 2113–2118. [Google Scholar]
- Pirow, R; Baumer, C; Paul, RJ. Crater landscape: Two-dimensional oxygen gradients in the circulatory system of the microcrustacean Daphnia magna. J. Exp. Biol 2004, 207, 4393–4405. [Google Scholar]
- Poole, DC; Behnke, BJ; McDonough, P; McAllister, RM; Wilson, DF. Measurement of muscle microvascular oxygen pressures: Compartmentalization of phosphorescent probe. Microcirculation 2004, 11, 317–326. [Google Scholar]
- Wilson, DF; Evans, SM; Jenkins, WT; Vinogradov, SA; Ong, E; Dewhirst, MW. Oxygen distributions within R3230Ac tumors growing in dorsal flap window chambers in rats. Adv. Exp. Med. Biol 1998, 454, 603–609. [Google Scholar]
- Wilson, DF; Lee, WMF; Makonnen, S; Apreleva, S; Vinogradov, SA. Oxygen pressures in the interstitial space of skeletal muscle and tumors in vivo. Oxygen Transp. Tiss 2008, 614, 53–62. [Google Scholar]
- Wilson, DF; Lee, WMF; Makonnen, S; Finikova, O; Apreleva, S; Vinogradov, SA. Oxygen pressures in the interstitial space and their relationship to those in the blood plasma in resting skeletal muscle. J. Appl. Physiol 2006, 101, 1648–1656. [Google Scholar]
- Wilson, DF; Vinogradov, SA; Dugan, BW; Biruski, D; Waldron, L; Evans, SA. Measurement of tumor oxygenation using new frequency domain phosphorometers. Com. Biochem. Phys. A 2002, 132, 153–159. [Google Scholar]
- Ziemer, LS; Lee, WMF; Vinogradov, SA; Sehgal, C; Wilson, DF. Oxygen distribution in murine tumors: characterization using oxygen-dependent quenching of phosphorescence. J. Appl. Physiol 2005, 98, 1503–1510. [Google Scholar]
- Yaseen, MA; Srinivasan, VJ; Sakadzic, S; Wu, W; Ruvinskaya, S; Vinogradov, SA; Boas, DA. Optical monitoring of oxygen tension in cortical microvessels with confocal microscopy. Opt. Express 2009, 17, 22341–22350. [Google Scholar]
- Dobrucki, JW. Interaction of oxygen-sensitive luminescent probes Ru(phen)(3)(2+) and Ru(bipy)(3)(2+) with animal and plant cells in vitro—Mechanism of phototoxicity and conditions for non-invasive oxygen measurements. J. Photoch. Photobio. B 2001, 65, 136–144. [Google Scholar]
- Kiernan, P; McDonagh, C; Maccraith, BD; Mongey, K. Ruthenium-doped sol-gel derived silica films: Oxygen sensitivity of optical decay times. J. Sol-Gel Sci. Tech 1994, 2, 513–517. [Google Scholar]
- Papkovsky, DB. Luminescent porphyrins as probes for optical (Bio)sensors. Sens. Actuat. B-Chem 1993, 11, 293–300. [Google Scholar]
- Hartmann, P; Trettnak, W. Effects of polymer matrices on calibration functions of luminescent oxygen sensors based on porphyrin ketone complexes. Anal. Chem 1996, 68, 2615–2620. [Google Scholar]
- Lavin, P; Mcdonagh, CM; Maccraith, BD. Optimization of Ormosil films for optical sensor applications. J. Sol.-Gel. Sci. Tech 1998, 13, 641–645. [Google Scholar]
- McDonagh, C; MacCraith, BD; McEvoy, AK. Tailoring of sol-gel films for optical sensing of oxygen in gas and aqueous phase. Anal. Chem 1998, 70, 45–50. [Google Scholar]
- Nock, V; Alkaisi, M; Blaikie, RJ. Photolithographic patterning of polymer-encapsulated optical oxygen sensors. Microelectron. Eng 2010, 87, 814–816. [Google Scholar]
- Ogurtsov, VI; Papkovsky, DB. Application of frequency spectroscopy to fluorescence-based oxygen sensors. Sens. Actuat. B-Chem 2006, 113, 608–616. [Google Scholar]
- Stucker, M; Schulze, L; Pott, G; Hartmann, P; Lubbers, DW; Rochling, A; Altmeyer, P. FLIM of luminescent oxygen sensors: clinical applications and results. Sens. Actuat. B-Chem 1998, 51, 171–175. [Google Scholar]
- Tripathi, VS; Kandimalla, VB; Ju, HX. Preparation of ormosil and its applications in the immobilizing biomolecules. Sens. Actuat. B-Chem 2006, 114, 1071–1082. [Google Scholar]
- Basu, BJ. Optical oxygen sensing based on luminescence quenching of platinum porphyrin dyes doped in ormosil coatings. Sens. Actuat. B-Chem 2007, 123, 568–577. [Google Scholar]
- Burke, CS; McGaughey, O; Sabattie, JM; Barry, H; McEvoy, AK; McDonagh, C; MacCraith, BD. Development of an integrated optic oxygen sensor using a novel, generic platform. Analyst 2005, 130, 41–45. [Google Scholar]
- Lee, YEK; Smith, R; Kopelman, R. Nanoparticle PEBBLE sensors in live cells and in vivo. Annu. Rev. Anal. Chem 2009, 2, 57–76. [Google Scholar]
- Lee, YEK; Kopelman, R. Optical nanoparticle sensors for quantitative intracellular imaging. Wires Nanomed. Nanobiol 2009, 1, 98–110. [Google Scholar]
- Vinogradov, SA; Lo, LW; Jenkins, WT; Evans, SM; Koch, C; Wilson, DF. Noninvasive imaging of the distribution in oxygen in tissue in vivo using near-infrared phosphors. Biophys. J 1996, 70, 1609–1617. [Google Scholar]
- Lebedev, AY; Cheprakov, AV; Sakadzic, S; Boas, DA; Wilson, DF; Vinogradov, SA. Dendritic phosphorescent probes for oxygen imaging in biological systems. ACS Appl. Mater. Inter 2009, 1, 1292–1304. [Google Scholar]
- Mehta, G; Lee, J; Cha, W; Tung, YC; Linderman, JJ; Takayama, S. Hard top soft bottom microfluidic devices for cell culture and chemical analysis. Anal. Chem 2009, 81, 3714–3722. [Google Scholar]
- Oppegard, SC; Blake, AJ; Williams, JC; Eddington, DT. Precise control over the oxygen conditions within the Boyden chamber using a microfabricated insert. Lab Chip 2010, 10, 2366–2373. [Google Scholar]
- Molter, TW; McQuaide, SC; Suchorolski, MT; Strovas, TJ; Burgess, LW; Meldrum, DR; Lidstrom, ME. A microwell array device capable of measuring single-cell oxygen consumption rates. Sens. Actuat. B-Chem 2009, 135, 678–686. [Google Scholar]
- Etzkorn, JR; Wu, WC; Tian, ZY; Kim, P; Jang, SH; Meldrum, DR; Jen, AK-Y; Parviz, BA. Using micro-patterned sensors and cell self-assembly for measuring the oxygen consumption rate of single cells. J. Micromech. Microeng 2010, 20, 095017. [Google Scholar]
- Ghajar, CM; Bissell, MJ. Tumor engineering: The other face of tissue engineering. Tiss. Eng. Pt. A 2010, 16, 2153–2156. [Google Scholar]
- Stroock, AD; Fischbach, C. Microfluidic culture models of tumor angiogenesis. Tiss. Eng. Pt. A 2010, 16, 2143–2146. [Google Scholar]







| Indicator | Encapsulation Matrix | Unquenched Lifetime (μs) | Quantum Yield | Reported Sensitivity* | Excitation Peaks (nm) | Emission Peaks (nm) | [Refs] |
|---|---|---|---|---|---|---|---|
| ([Ru(dpp)3]2+ | Polystyrene | 5 | NR | 22% signal decrease from N2 to air | 450 | 600 | [60] |
| ([Ru(dpp)3]2+ | Plasticized PVC | 5 | NR | 50% signal decrease from N2 to air | 450 | 600 | [60] |
| ([Ru(dpp)3]2+ | None | 6.3 at 23 °C (silicone-soluble ion pair in 2-butanone ) | 0.3 (in water/ethanol) 0.35 (silicone-soluble ion pair in 2-butanone ) | kQ(dissolved O2) = 2.5 (109dm−3 mol−1s−1) (in methanol) | 460 | 613, 627 | [37,92] |
| ([Ru(phen)3]2+ | None | 0.74 at 23 °C (silicone-soluble ion pair in 2-butanone ) | 0.08 (silicone-soluble ion pair in 2-butanone ) | kQ(dissolved O2) = 4.2 (109dm−3 mol−1s−1) | 447,421 | 605, 625 | [37,92] |
| [Ru(Ph2phen)3]2+ | Sol-gel silica | 5.8 | NR | τ N2/τO2 = 5 | NR | NR | [123] |
| ([Ru(bpy)3]2+ | None | 0.6 | 0.042 | kQ(dissolved O2)=3.3 (109dm−3 mol−1s−1) | 423, 452 | 613, 627 | [37,83] |
| ([Ru(bpy)3]2+ | Sol-gel silica | 1.26 | NR | τ N2/τO2 = 2 | NR | NR | [123] |
| Indicator | Encapsulation Matrix | Unquenched Lifetime (μs) | Quantum Yield | Reported Sensitivity * | Excitation Peaks (nm) | Emission Peaks (nm) | [Refs] |
| PtOEPK | Polystyrene | 61.4 at 22°C | 0.12 | High | 398, 592 | 759 | [61] |
| PtOEPK | PDMA | NR | NR | QDO = 97.5% | NR | 754 | [56] |
| PdOEPK | Polystyrene | 480 at 22°C | 0.01 | Very high | 410, 602 | 790 | [61] |
| PtOEP | Polystyrene | 94.7 at 20°C | NR | τ0/τair = 3.60 | 383, 535 | 647 | [60,98] |
| Pd-coproporphyrin | None (aqueous solution) | 530 (no BSA), 1200 (BSA) | 0.2 | kQ = 195 mmHg−1s−1 | 393. 545 | 667 | [83,124] |
| Pt-coproporphyrin | None (aqueous solution) | 100 | 0.4 | NR | 380, 535 | 650 | [124] |
| Pd-meso-tetra-(4-carboxy- phenyl) tetrabenzoporphyrin-dendrimer (Oxyphor G2) | None (BSA solution at pH 6.8, 23.5 °C) | 276 | 0.12 | kQ = 195 mmHg−1s−1 | 442, 632 | 800 | [106,109] |
| Pd-meso-tetra-(4-carboxyphenyl) porphyrin-dendrimer (Oxyphor R2) | None (BSA solution at pH 6.8, 23.5 °C) | 738 | 0.1 | kQ = 270 mmHg−1s−1 | 415, 524 | 700 | [109] |
| Pd-meso-tetra (4-Carboxyphenyl) Porphine (Oxyphor R0) | None (albumin solution at pH 6.8, 23 °C) | 705 | 0.06 | kQ = 246 mmHg−1s−1 | 416, 523 | 687 | [106] |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grist, S.M.; Chrostowski, L.; Cheung, K.C. Optical Oxygen Sensors for Applications in Microfluidic Cell Culture. Sensors 2010, 10, 9286-9316. https://doi.org/10.3390/s101009286
Grist SM, Chrostowski L, Cheung KC. Optical Oxygen Sensors for Applications in Microfluidic Cell Culture. Sensors. 2010; 10(10):9286-9316. https://doi.org/10.3390/s101009286
Chicago/Turabian StyleGrist, Samantha M., Lukas Chrostowski, and Karen C. Cheung. 2010. "Optical Oxygen Sensors for Applications in Microfluidic Cell Culture" Sensors 10, no. 10: 9286-9316. https://doi.org/10.3390/s101009286
APA StyleGrist, S. M., Chrostowski, L., & Cheung, K. C. (2010). Optical Oxygen Sensors for Applications in Microfluidic Cell Culture. Sensors, 10(10), 9286-9316. https://doi.org/10.3390/s101009286