Quantum Cascade Laser-Based Photoacoustic Spectroscopy for Trace Vapor Detection and Molecular Discrimination
Abstract
:1. Introduction
2. Experimental Section
2.1. Quantum Cascade Laser
2.2. MEMS-Scale Photoacoustic Cell
2.3. Sample Generation
2.4. Data Acquisition
2.5. Instrumentation
2.6. Software
3. Results and Discussion
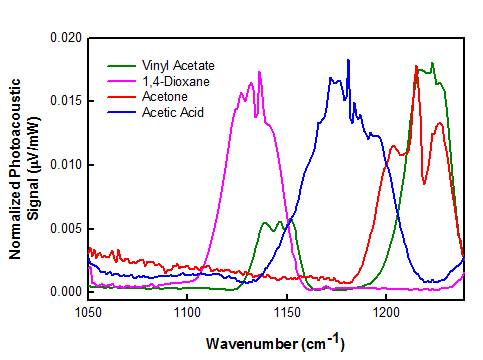
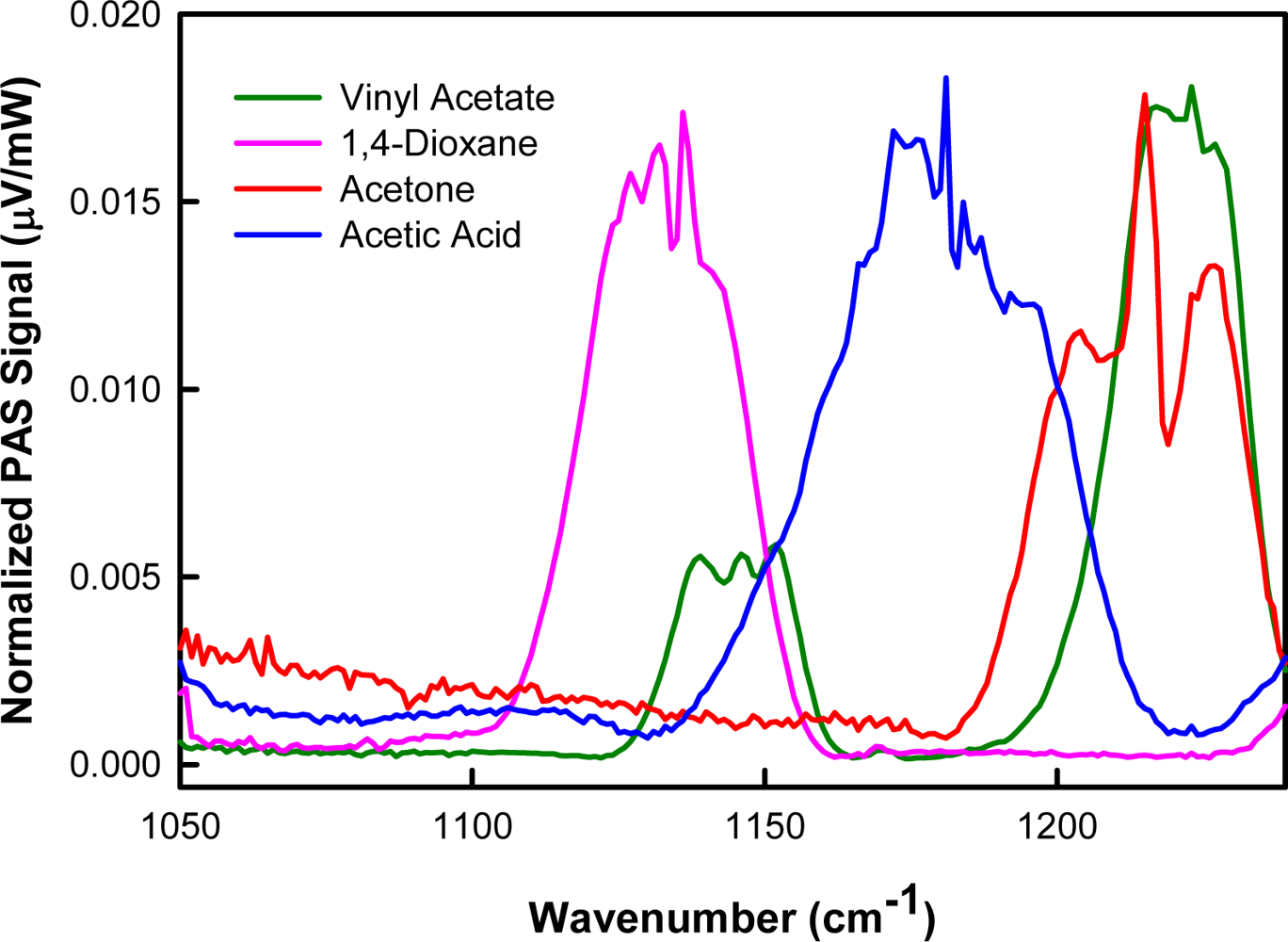
3.1. Spectroscopic Data
3.2. Sensor Responsivity
3.3. Partial Least Squares Regression Analysis
4. Conclusions
Acknowledgments
References and Notes
- Nägele, M.; Sigrist, M.W. Mobile laser spectrometer with novel resonant multipass photoacoustic cell for trace-gas sensing. Appl. Phys. B 2001, 70, 895–901. [Google Scholar]
- Bijnen, F.G.C.; Reuss, J.; Harren, F.J.M. Geometrical optimization of a longitudinal photoacoustic cell for a sensitive and fast trace gas detection. Rev. Sci. Instrum 1996, 67, 2914–2933. [Google Scholar]
- Firebaugh, S.L.; Jensen, K.F.; Schmidt, M.A. Miniaturization and integration of photoacoustic detection with a microfabricated chemical reactor system. JMEMS 2001, 10, 232–237. [Google Scholar]
- Firebaugh, S.L.; Jensen, K.F.; Schmidt, M.A. Miniaturization and integration of photoacoustic detection. J. Appl. Phys 2002, 92, 1555–1563. [Google Scholar]
- Pellegrino, P.; Polcawich, R. Advancement of a MEMS photoacoustic chemical sensor. Proc. SPIE 2003, 5085, 52–63. [Google Scholar]
- Pellegrino, P.; Polcawich, R.; Firebaugh, S.L. Miniature photoacoustic chemical sensor using microelectromechanical structures. Proc. SPIE 2004, 5416, 42–53. [Google Scholar]
- Heaps, D.A.; Pellegrino, P. Examination of a quantum cascade laser source for a MEMS-scale photoacoustic chemical sensor. Proc. SPIE 2006, 6218, 621805-1–621805-9. [Google Scholar]
- Heaps, D.A.; Pellegrino, P. Investigations of intraband quantum cascade laser source for a MEMS-scale photoacoustic sensor. Proc. SPIE 2007, 6554, 65540F-1–65540F-9. [Google Scholar]
- Bonne, U.; Cole, B.E.; Higashi, R.E. Micromachined integrated opto-flow gas/liquid sensor. US Patent 5886249,. March 23 1999. [Google Scholar]
- Holthoff, E.L.; Heaps, D.A.; Pellegrino, P.M. Development of a MEMS-Scale photoacoustic chemical sensor using a quantum cascade laser. IEEE Sens. J 2010, 10, 572–577. [Google Scholar]
- Patel, C.K.N.; Tam, A.C. Pulsed optoacoustic spectroscopy of condensed matter. Rev. Mod. Phys 1981, 53, 517–550. [Google Scholar]
- Kinney, J.B.; Staley, R.H. Applications of photoacoustic spectroscopy. Ann. Rev. Mater. Sci 1982, 12, 295–321. [Google Scholar]
- Tam, A.C. Applications of photoacoustic sensing techniques. Rev. Mod. Phys 1986, 58, 381–431. [Google Scholar]
- Polcawich, R.G.; Pellegrino, P.M. Microelectromechanical resonant photoacoustic cell. US Patent 7304732,. August 19 2003. [Google Scholar]
- Miklós, A.; Hess, P.; Mohácsi, A.; Sneider, J.; Kamm, S.; Schafer, S. Improved photoacoustic detector for monitoring polar molecules such as ammonia with a 1.53 μm DFB diode laser. proceedings of Photoacoustic and Photothermal Phenomena: 10th International Conference; Scudieri, F., Bertolotti, M., Eds.; 1999. [Google Scholar]
- Vibrational and/or electronic energy levels. Available online: www.webbook.nist.gov (accessed on February 26, 2010).
- McMurry, J. Organic Chemistry, 5th ed; Brooks/Cole: Pacific Grove, CA, USA, 1999. [Google Scholar]
- Chemical Sampling Information: Acetic Acid. Available online: www.OSHA.gov (accessed on February 26, 2010).
- Chemical Sampling Information: Acetone. Available online: www.OSHA.gov (accessed on February 27, 2010).
- Chemical Sampling Information: Dioxane. Available online: www.OSHA.gov (accessed on February 25, 2010).
- Chemical Sampling Information: Vinyl Acetate. Available online: www.OSHA.gov (accessed on February 26, 2010).
- Beebe, K.R.; Pell, R.J.; Seasholtz, M.B. Chemometrics: A Practical Guide; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Esbensen, K.H. Multivariate Data Analysis - In Practice, 5th ed; CAMO Process AS: Oslo, Norway, 2004. [Google Scholar]











| Analyte | NIOSH Threshold Limit Value |
|---|---|
| Acetic Acid | 10 ppm (TWA)1 |
| Acetone | 250 ppm2 |
| 1,4-Dioxane | 1 ppm (C)3 |
| Vinyl Acetate | 4 ppm (CL)4 |
| Factor # | Percent Concentration Variance | Percent Spectral Variance | ||
|---|---|---|---|---|
| Each Factor | Cumulative | Each Factor | Cumulative | |
| 1 | 35.20 | 35.20 | 91.14 | 91.14 |
| 2 | 26.76 | 61.96 | 7.43 | 98.57 |
| 3 | 12.60 | 74.56 | 1.28 | 99.85 |
| 4 | 24.58 | 99.14 | 0.13 | 99.98 |
| 5 | 0.25 | 99.39 | 0.01 | 99.99 |
| 6 | 0.23 | 99.62 | 0.01 | 100.00 |
| 7 | 0.15 | 99.77 | 0.00 | 100.00 |
| 8 | 0.08 | 99.85 | 0.00 | 100.00 |
| 9 | 0.06 | 99.91 | 0.00 | 100.00 |
| 10 | 0.02 | 99.93 | 0.00 | 100.00 |
| Analyte | Slope | Offset | R2 | RMSEC | RMSEP | |||
|---|---|---|---|---|---|---|---|---|
| Calibration | Prediction | Calibration | Prediction | Calibration | Prediction | |||
| Acetic Acid | 0.9911 | 0.9872 | 0.0036 | 0.0051 | 0.9911 | 0.9899 | 0.1216 | 0.1286 |
| Acetone | 0.9798 | 0.9864 | 0.0127 | 0.0077 | 0.9798 | 0.9773 | 0.2568 | 0.2711 |
| 1,4-Dioxane | 0.9933 | 0.9877 | 0.0045 | 0.0066 | 0.9933 | 0.9922 | 0.1494 | 0.1615 |
| Vinyl Acetate | 0.9984 | 1.0024 | 0.0012 | −0.0012 | 0.9984 | 0.9982 | 0.0834 | 0.0905 |
| Sample # | Component Actual Concentration (ppm) | Acetic Acid Algorithm Prediction | Acetone Algorithm Prediction | 1,4-Dioxane Algorithm Prediction | Vinyl Acetate Algorithm Prediction | |||
|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Acetone | 1,4-Dioxane | Vinyl Acetate | Concentration (ppm) | Concentration (ppm) | Concentration (ppm) | Concentration (ppm) | |
| 1 | 0.000 | 0.000 | 1.590 | 0.000 | 0.069 | 0.166 | 1.746 | 0.030 |
| 2 | 0.000 | 0.000 | 0.000 | 1.131 | 0.000 | 0.000 | 0.000 | 1.060 |
| 3 | 1.114 | 0.000 | 0.000 | 0.000 | 1.046 | 0.215 | 0.050 | 0.016 |
| 4 | 0.000 | 1.566 | 0.000 | 0.000 | 0.055 | 1.146 | 0.114 | 0.061 |
| 5 | 1.671 | 0.000 | 0.000 | 2.827 | 1.864 | 0.457 | 0.029 | 2.683 |
| 6 | 1.671 | 2.350 | 0.000 | 0.000 | 1.716 | 2.294 | 0.016 | 0.000 |
| 7 | 1.671 | 2.350 | 0.000 | 2.827 | 1.824 | 2.573 | 0.000 | 2.500 |
| 8 | 0.000 | 2.350 | 0.000 | 2.827 | 0.000 | 2.747 | 0.000 | 2.586 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Holthoff, E.; Bender, J.; Pellegrino, P.; Fisher, A. Quantum Cascade Laser-Based Photoacoustic Spectroscopy for Trace Vapor Detection and Molecular Discrimination. Sensors 2010, 10, 1986-2002. https://doi.org/10.3390/s100301986
Holthoff E, Bender J, Pellegrino P, Fisher A. Quantum Cascade Laser-Based Photoacoustic Spectroscopy for Trace Vapor Detection and Molecular Discrimination. Sensors. 2010; 10(3):1986-2002. https://doi.org/10.3390/s100301986
Chicago/Turabian StyleHolthoff, Ellen, John Bender, Paul Pellegrino, and Almon Fisher. 2010. "Quantum Cascade Laser-Based Photoacoustic Spectroscopy for Trace Vapor Detection and Molecular Discrimination" Sensors 10, no. 3: 1986-2002. https://doi.org/10.3390/s100301986