Mid-Infrared Fiber-Coupled Photoacoustic Sensor for Biomedical Applications
Abstract
:1. Introduction
2. MIR Fibers
3. Photoacoustic Spectroscopy
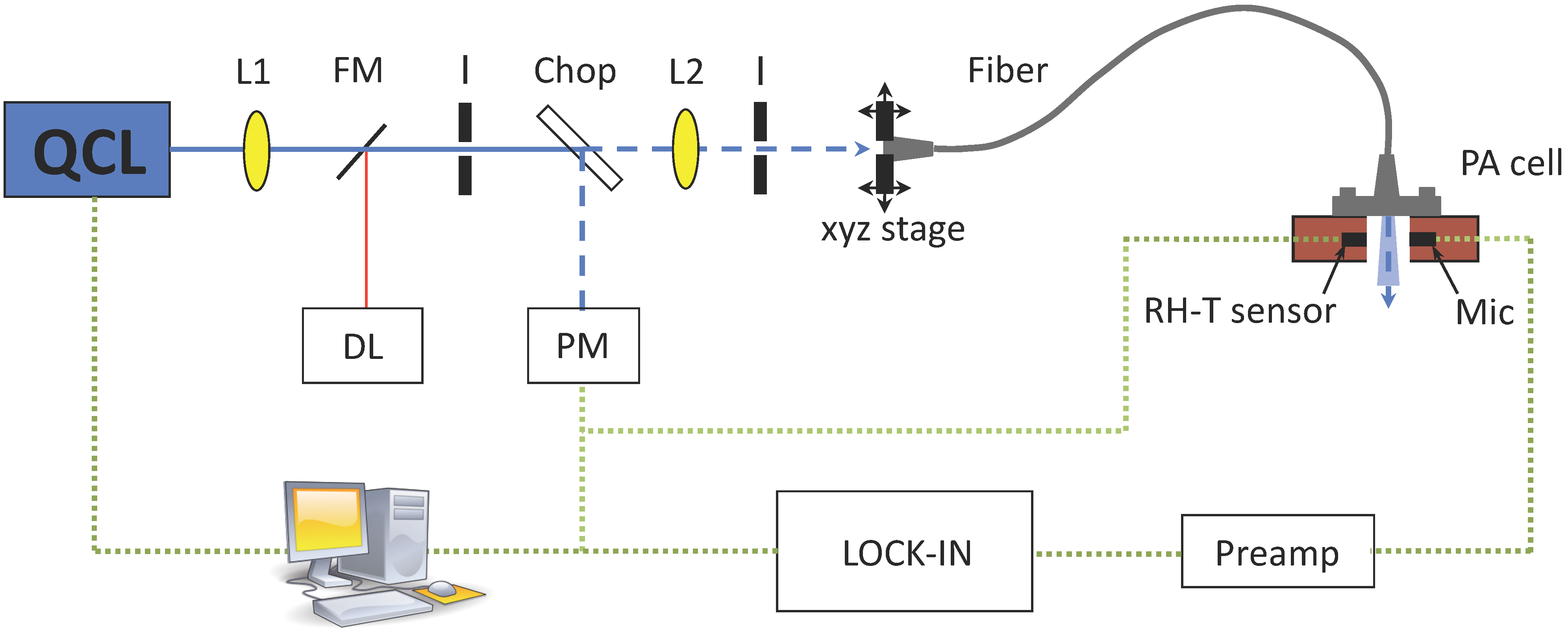
4. Experimental Section
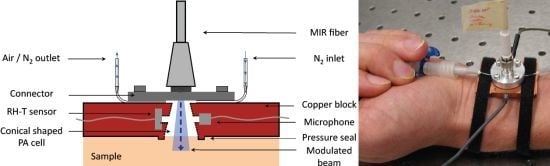
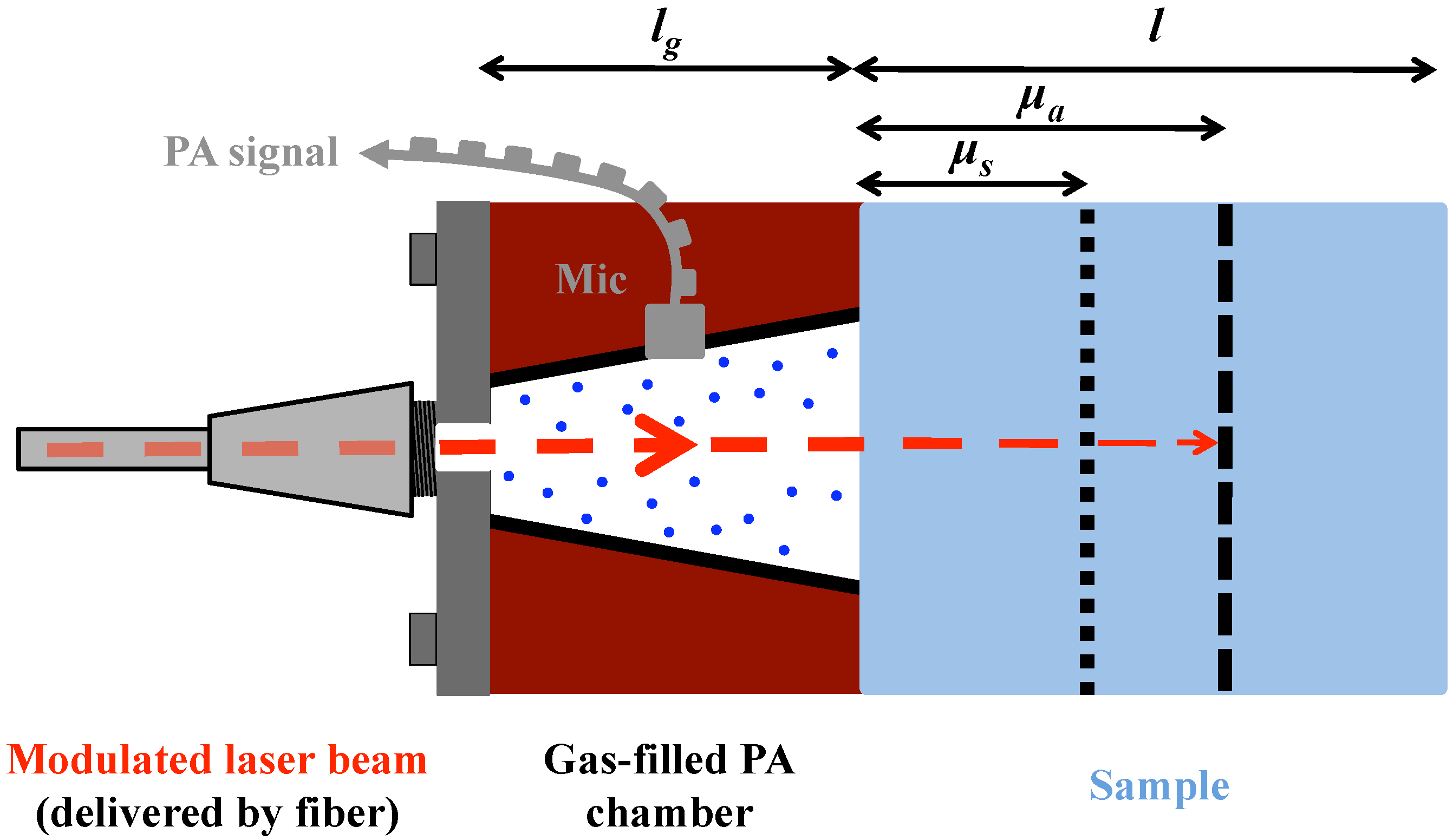
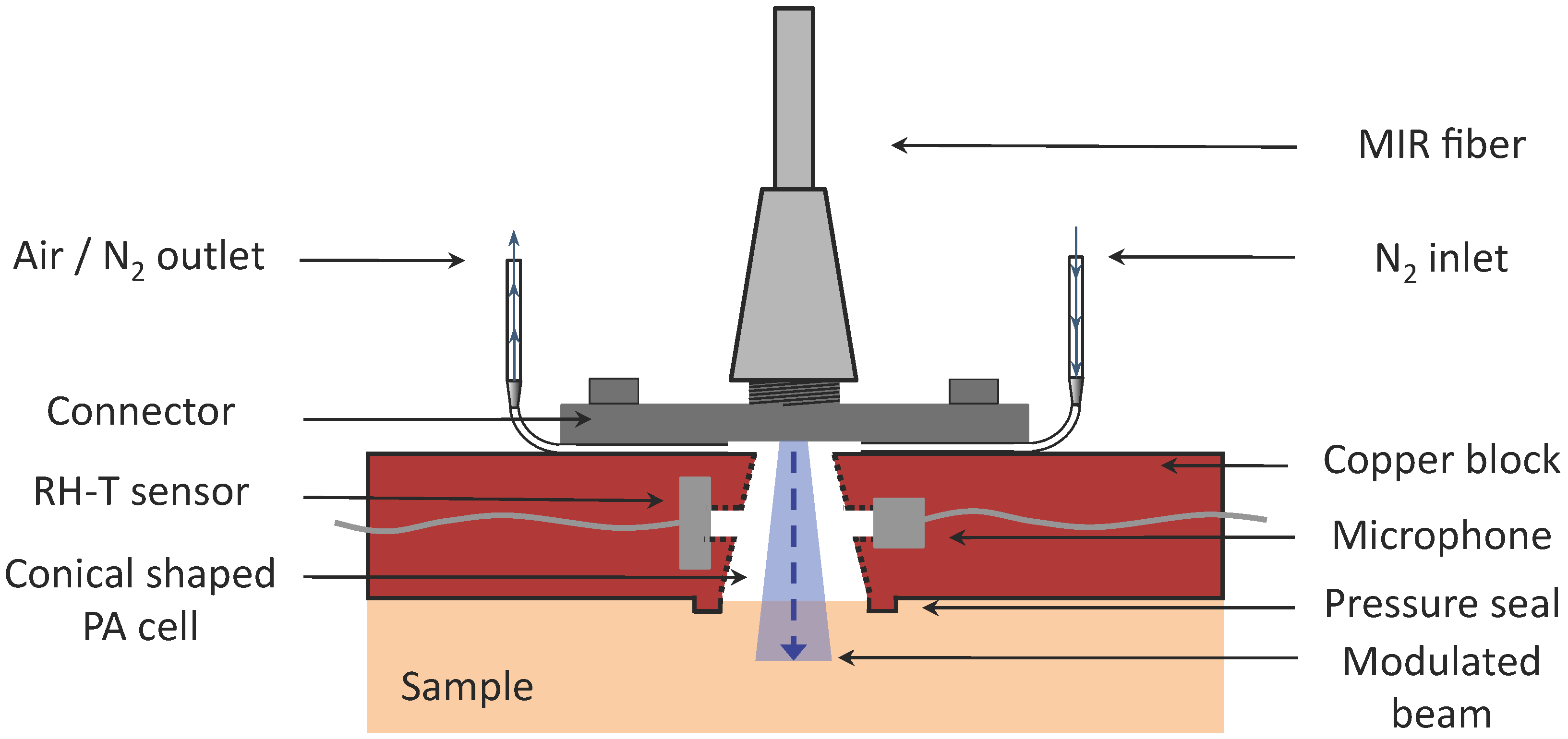
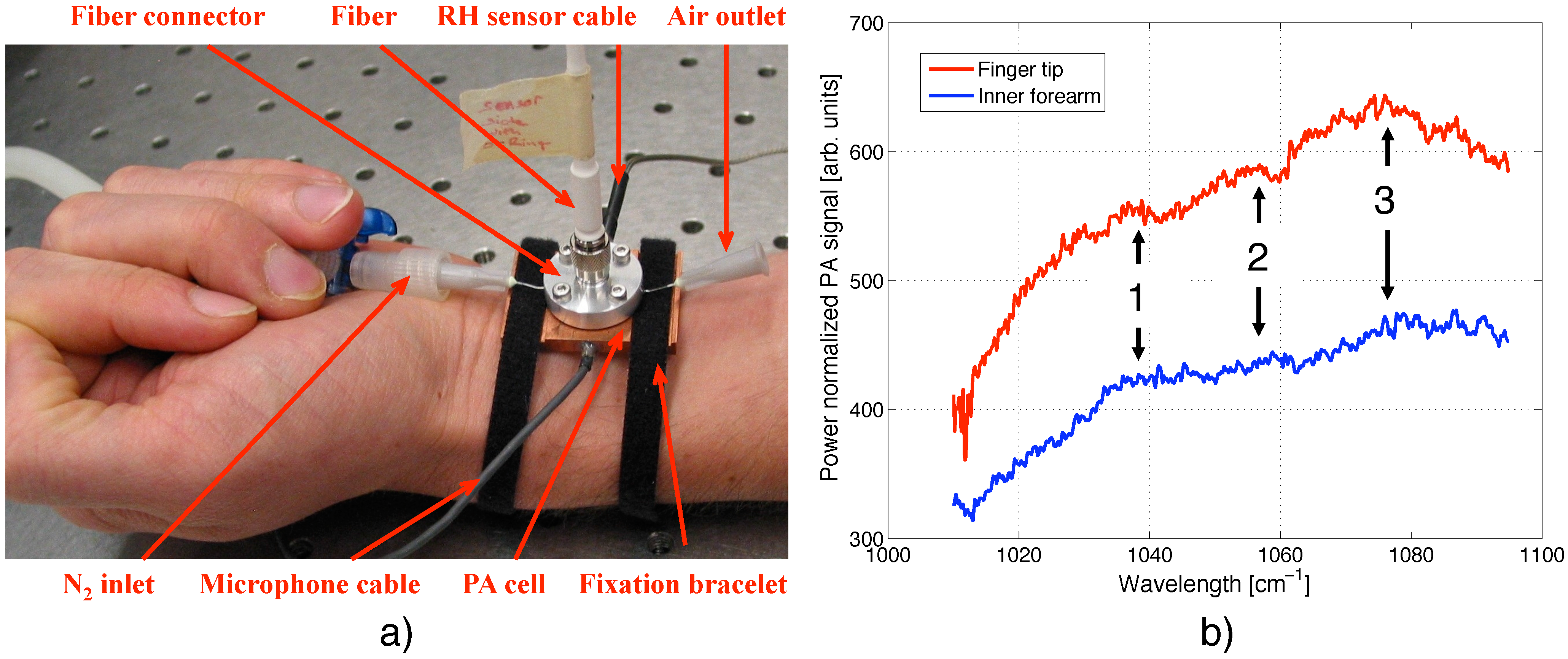
4.1. Photoacoustic Cell Design
5. Results and Discussion
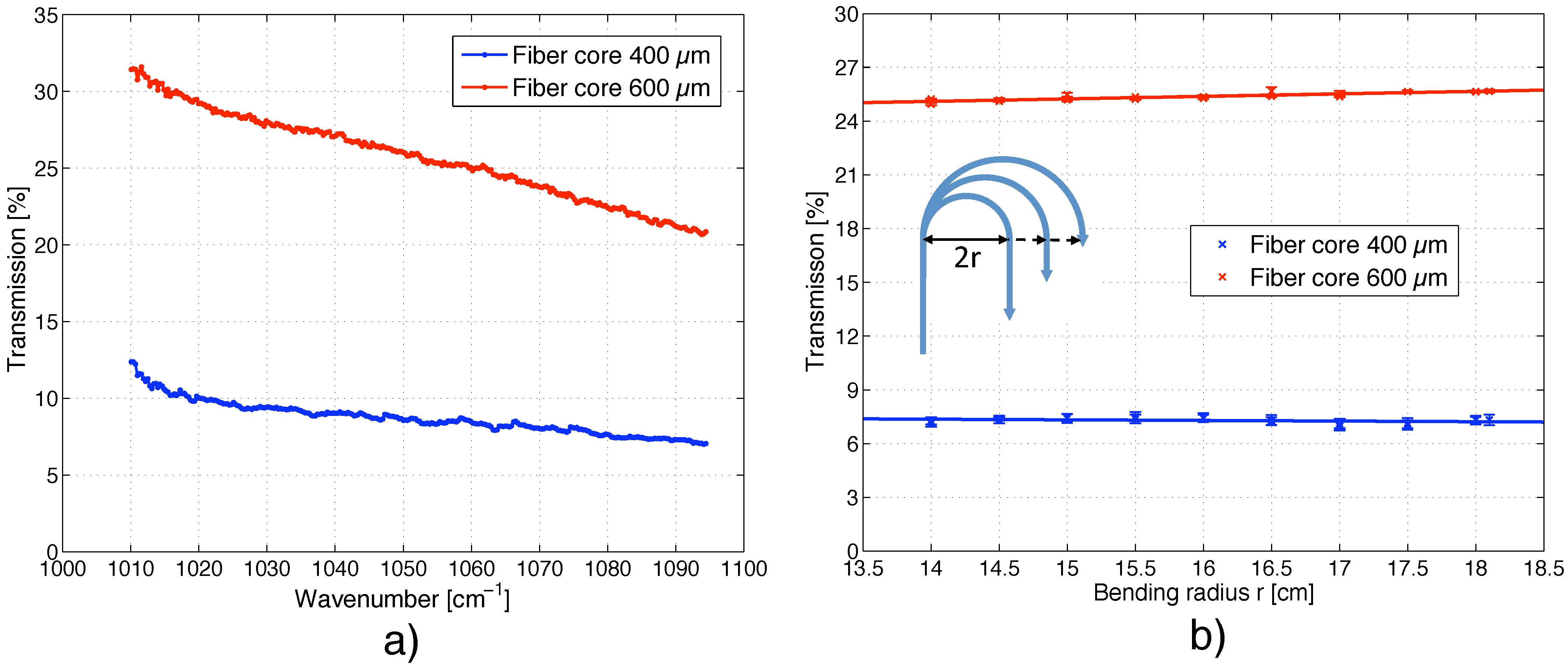
5.1. Transmission of the MIR Fiber
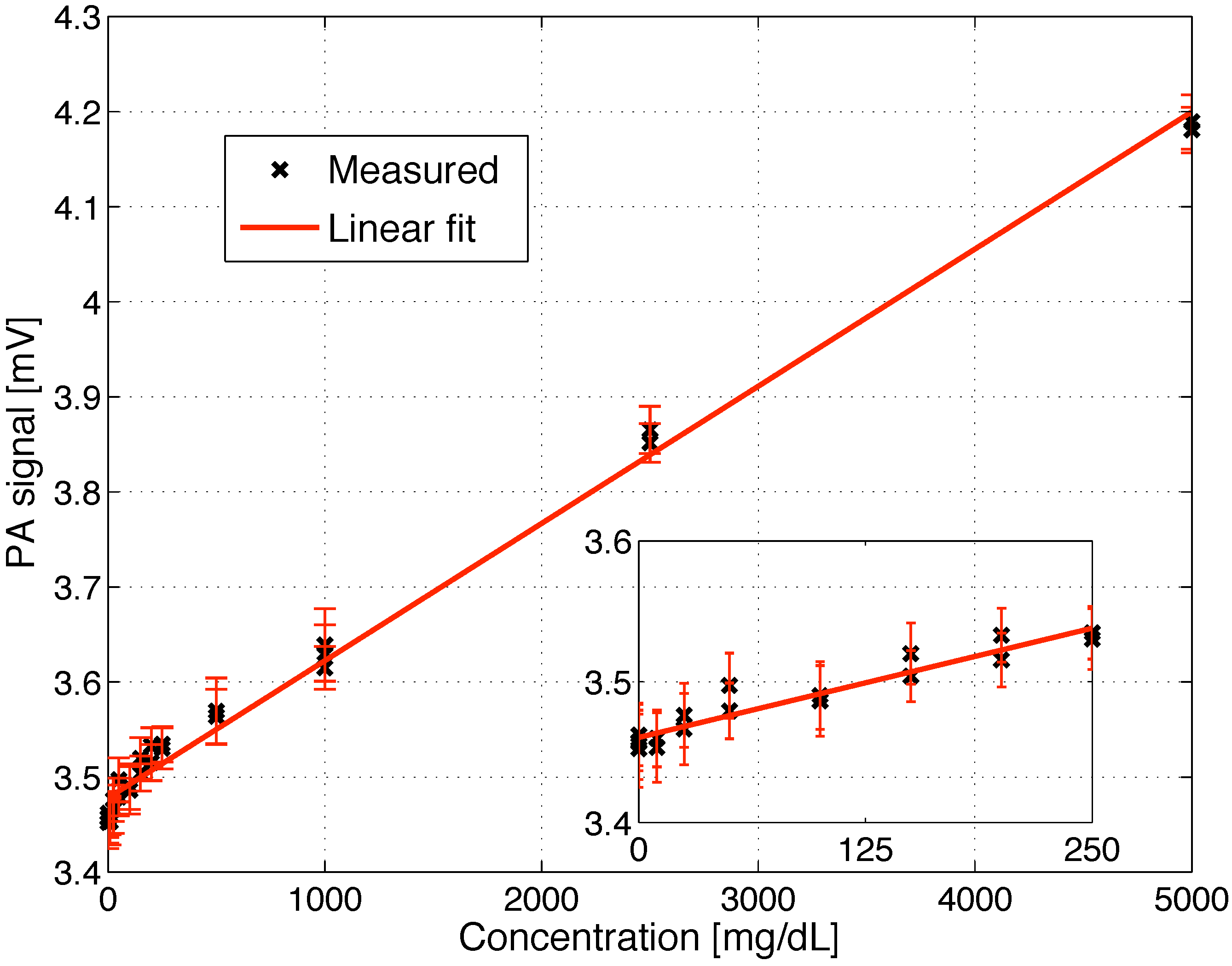
5.2. Glucose Detection in Aqueous Solutions
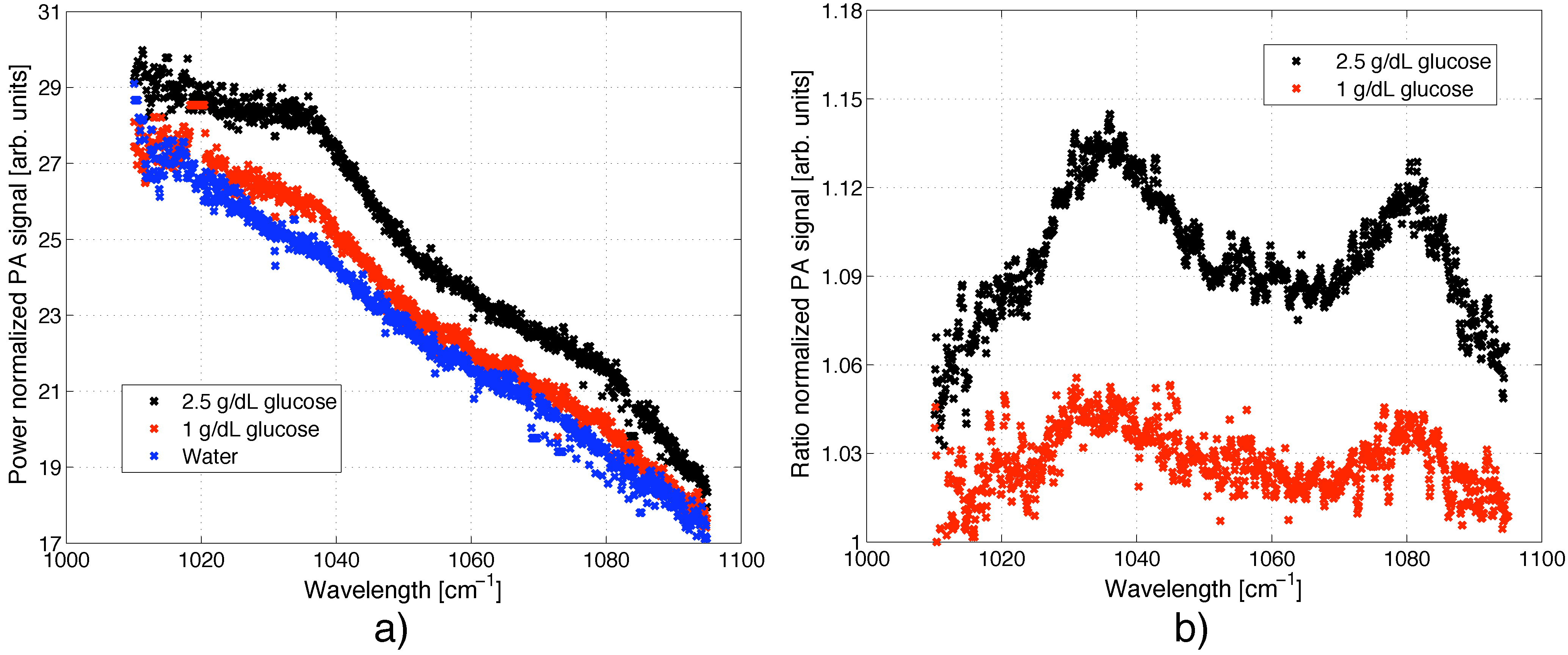
5.3. Spectra of Glucose in Aqueous Solutions
5.4. In vivo Spectra of Human Skin
6. Conclusions
Acknowledgments
References
- Bindig, U.; Meinke, M.; Gersonde, I.; Spector, O.; Vasserman, I.; Katzir, A.; Müller, G. IR-biosensor: Flat silver halide fiber for bio-medical sensing? Sens. Actuators B 2001, 74, 37–46. [Google Scholar]
- Farahi, R.H.; Passian, A.; Tetard, L.; Thundat, T. Pump-probe photothermal spectroscopy using quantum cascade lasers. J. Phys. D: Appl. Phys. 2012, 45, 125101. [Google Scholar]
- Moser, F.; Bunimovich, D.; DeRowe, A.; Eyal, O.; German, A.; Gotshal, Y.; Levite, A.; Nagli, L.; Ravid, A.; Scharf, V.; et al. Medical applications of infrared transmitting silver halide fibers. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 872–879. [Google Scholar]
- Merberg, G.N. Current status of infrared fiber optics for medical laser power delivery. Lasers Surg. Med. 1993, 13, 572–576. [Google Scholar]
- Gal, D.; Katzir, A. Silver halide optical fibers for medical applications. IEEE J. Quantum Electron. 1987, QE-23, 1827–1835. [Google Scholar]
- Beenen, A.; Niessner, R. Development of a photoacoustic trace gas sensor based on fiber-optically coupled NIR laser diodes. Appl. Spec. 1999, 53, 1040–1044. [Google Scholar]
- Mohacsi, A.; Bozoki, Z.; Niessner, R. Direct diffusion sampling-based photoacoustic cell for in situ and on-line monitoring of benzene and toluene concentration in water. Sens. Actuators B 2001, 79, 127–131. [Google Scholar]
- Schilt, S.; Besson, J.P.; Thévenaz, L. Fibre-coupled photoacoustic sensor for sub-ppm methane monitoring. Proc. SPIE 2004, 5502, 317–320. [Google Scholar]
- Elia, A.; Spagnolo, V.; Franco, C.D.; Lugara, P.M.; Scamarcio, G. Trace gas sensing using quantum cascade lasers and a fiber-coupled optoacoustic sensor: Application to formaldehyde. J. Phys. Conf. Series 2010, 214, 012037. [Google Scholar]
- Kottmann, J.; Rey, J.M.; Sigrist, M.W. New photoacoustic cell with diamond window for mid-infrared investigations on biological samples. Proc. SPIE 2012, 8223, 82231A. [Google Scholar]
- Kottmann, J.; Rey, J.M.; Luginbühl, J.; Reichmann, E.; Sigrist, M.W. Glucose sensing in human epidermis using mid-infrared photoacoustic detection. Biomed. Opt. Exp. 2012, 3, 667–680. [Google Scholar]
- Raichlin, Y.; Katzir, A. Fiber-optic evanescent wave spectroscopy in the middle infrared. Appl. Spec. 2008, 62, 55A–72A. [Google Scholar]
- Shalem, S.; Katzir, A. Core-clad silver halide fibers with few modes and a broad transmission in the mid-infrared. Opt. Lett. 2005, 30, 1929–1931. [Google Scholar]
- Rave, E.; Nagli, L.; Katzir, A. Ordered bundles of infrared-transmitting AgClBr fibers: Optical characterization of individual fibers. Opt. Lett. 2000, 25, 1237–1239. [Google Scholar]
- Lewi, T.; Shalem, S.; Tsun, A.; Katzir, A. Silver halide single-mode fibers with improved properties in the middle infrared. Appl. Phys. Lett. 2007, 91, 251112. [Google Scholar]
- Millo, A.; Lobachinsky, L.; Katzir, A. Single-mode octagonal photonic crystal fibers for the middle infrared. Appl. Phys. Lett. 2008, 92, 021112. [Google Scholar]
- Lewi, T.; Tsun, A.; Katzir, A.; Kaster, J.; Fuchs, F. Silver halide single mode fibers for broadband middle infrared stellar interferometry. Appl. Phys. Lett. 2009, 94, 261105. [Google Scholar]
- Heise, H.M.; Bittner, A.; Küpper, L.; Butvina, L.N. Comparison of evanescent wave spectroscopy based on silver halide fibers with conventional ATR-IR spectroscopy. J. Mol. Struct. 1997, 410, 521–525. [Google Scholar]
- Kriesel, J.M.; Gat, N.; Bernacki, B.E.; Erikson, R.L.; Cannon, B.D.; Myers, T.L.; Bledt, C.M.; Harrington, J.A. Hollow core fber optics for mid-wave and long-wave infrared spectroscopy. Proc. SPIE 2011, 8018, 80180V. [Google Scholar]
- Harrington, J.A.; Bledt, C.M.; Kriesel, J.M. Hollow waveguide for the transmission of quantum cascade laser (QCL) energy for spectroscopic applications. Proc. SPIE 2011, 7894, 789414. [Google Scholar]
- Patimisco, P.; Spagnolo, V.; Vitiello, M.S.; Tredicucci, A.; Scamarcio, G.; Bledt, C.M.; Harrington, J.A. Coupling external cavity mid-IR quantum cascade lasers with low loss hollow metallic/dielectric waveguides. Appl. Phys. B 2012, 108, 255–260. [Google Scholar]
- Spagnolo, V.; Patimisco, P.; Borri, S.; Scamarcio, G.; Bernacki, B.E.; Kriesel, J. Part-per-trillion level SF6 detection using a quartz enhanced photoacoustic spectroscopy-based sensor with single-mode fiber-coupled quantum cascade laser excitation. Opt. Lett. 2012, 37, 4461–4463. [Google Scholar]
- Rosencwaig, A.; Gersho, A. Theory of photoacoustic effect with solids. J. Appl. Phys. 1976, 47, 64–69. [Google Scholar]
- Tam, A.C. Ultrasensitive Laser Spectroscopy; Academic Press Inc.: Waltham, MA, USA, 1983; Chapter 1; pp. 1–108. [Google Scholar]
- Kottmann, J.; Rey, J.M.; Sigrist, M.W. New photoacoustic cell design for studying aqueous solutions and gels. Rev. Sci. Instrum. 2011, 82, 084903. [Google Scholar]
- Moser, F.; Barkay, N.; Levite, A.; Margalit, E.; Paiss, I.; Saar, A.; Schnitzer, I.; Zur, A.; Katzir, A. Research and development on silver halide fibers at Tel Aviv University. Proc. SPIE 1990, 1228, 128–139. [Google Scholar]
- Israeli, S.; Katzir, A. Optical losses of AgClBr crystals in the middle infrared. Opt. Mater. 2011, 33, 1825–1828. [Google Scholar]
- DKE, D.V. Optische Strahlensicherheit und Laser 1 und 2; DIN Deutsches Institut für Normung e. V.: Berlin, Germany, 2007. [Google Scholar]
- Günzler, H.; Gremlich, H.U. IR-Spektroskopie; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Pleitez, M.; von Lilienfeld-Toal, H.; Mäntele, W. Infrared spectroscopic analysis of human interstitial fluid in vitro and in vivo using FT-IR spectroscopy and pulsed quantum cascade lasers (QCL): Establishing a new approach to noninvasive glucose measurement. Spectrochim. Acta Part A 2012, 85, 61–65. [Google Scholar]
- Garidel, P. Mid-FTIR-Microspectroscopy of stratum corneum single cells and stratum corneum tissue. Phys. Chem. Chem. Phys. 2002, 4, 5671–5677. [Google Scholar]
- Lucassen, G.W.; van Veen, G.N.A.; Jansen, J.A.J. Band analysis of hydrated human skin stratum corneum attenuated total reflectance fourier transform infrared spectra in vivo. J. Biomed. Opt. 1998, 3, 267–280. [Google Scholar]
- Barry, R.W.; Edwards, H.G.M.; Williams, A.C. Fourier transform raman and infrared vibrational study of human skin: Assignment of spectral bands. J. Raman Spectrosc. 1992, 23, 641–645. [Google Scholar]
| Frequency [cm−1] | Assignment | Strength | Contribution |
|---|---|---|---|
| 1020 | albumin absorption | vw | - |
| 1034 | α & β D-glucose absorption | m | 1 |
| 1035 | ν(CC) skeletal cis conformation | m | 1 |
| 1047 | ν(C-OP) | w | 2 |
| 1052 | albumin absorption | w | 2 |
| 1054 | α D-glucose absorption | vw | 2 |
| 1077 | ν(CC) skeletal trans conformation | m | 3 |
| 1080 | symmetric | m | 3 |
| 1080 | β D-glucose absorption | m | 3 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kottmann, J.; Grob, U.; Rey, J.M.; Sigrist, M.W. Mid-Infrared Fiber-Coupled Photoacoustic Sensor for Biomedical Applications. Sensors 2013, 13, 535-549. https://doi.org/10.3390/s130100535
Kottmann J, Grob U, Rey JM, Sigrist MW. Mid-Infrared Fiber-Coupled Photoacoustic Sensor for Biomedical Applications. Sensors. 2013; 13(1):535-549. https://doi.org/10.3390/s130100535
Chicago/Turabian StyleKottmann, Jonas, Urs Grob, Julien M. Rey, and Markus W. Sigrist. 2013. "Mid-Infrared Fiber-Coupled Photoacoustic Sensor for Biomedical Applications" Sensors 13, no. 1: 535-549. https://doi.org/10.3390/s130100535