Sol-Gel Material-Enabled Electro-Optic Polymer Modulators
Abstract
:1. Introduction



2. Material Loss in Sol-Gel Materials
| Wavelength (nm) | Assignment |
|---|---|
| 1150 | CH[CH] third overtone |
| 1200 | CH[CH] third overtone |
| 1380 | CH[CH]+δ SiOSi second overtone |
| 1420 | CH[CH]+δ SiOSi second overtone |
| 1300–1600 | Si-OH and Zr-OH |
| 1630 | CH[CH=CH] second overtone |
| Chemical Name | Chemical Structure | [46] | Benefits | Drawbacks |
|---|---|---|---|---|
| Methacryl- oxypropyl- trimethoxysilane (MAPTMS) |  | 1.4277 | Better adhesion, photo-patternable | increases loss |
| Diphenyl- dimethoxysilane (DPDMS) | 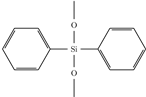 | 1.5447 | increases index, decreases loss | |
| Phenyl- trimethoxysilane (PTMS) | 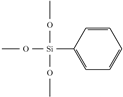 | 1.4734 | increases index, decreases loss | |
| Bis-pentafluoro- diphenyl- dimethoxysilane (BPFDPDMS) |  | 1.4181 | lowers index, decreases loss | reduces photo-sensitivity |
| Zirconium(IV) propoxide (ZPO) |  | n/a | increases index | increases viscosity, can cause gelation |



3. Adjusting the Refractive Index in Sol-Gel Materials


4. Sol-Gel Materials for Improved Poling


5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Brinker, C.F.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Klein, L. Sol-Gel Optics: Processing and Applications; Kluwer Academic Publisher: Hingham, MA, USA, 1994. [Google Scholar]
- Wooten, E.L.; Kissa, K.M.; Yi-Yan, A.; Murphy, E.J.; Lafaw, D.A.; Hallemeier, P.F.; Maack, D.; Attanasio, D.V.; Fritz, D.J.; McBrien, G.J.; et al. A review of lithium niobate modulators for fiber-optic communications systems. IEEE J. Sel. Top. Quantum Electron. 2000, 6, 69–82. [Google Scholar] [CrossRef]
- Zelmon, D.E.; Small, D.L.; Jundt, D. Infrared corrected sellmeier coefficients for congruently grown lithium niobate and 5 mol.% magnesium oxide-doped lithium niobate. J. Opt. Soc. Am. B 1997, 14, 3319–3322. [Google Scholar]
- Findakly, T.; Bramson, M. High-performance integrated-optical chip for a broad range of fiber-optic gyro applications. Opt. Lett. 1990, 15, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Treyz, G.; May, P.; Halbout, J.M. Silicon Mach–Zehnder waveguide interferometers based on the plasma dispersion effect. Appl. Phys. Lett. 1991, 59, 771–773. [Google Scholar] [CrossRef]
- Feng, N.N.; Liao, S.; Feng, D.; Dong, P.; Zheng, D.; Liang, H.; Shafiiha, R.; Li, G.; Cunningham, J.E.; Krishnamoorthy, A.V.; et al. High speed carrier-depletion modulators with 1.4 V-cm V(pi)L integrated on 0.25 microm silicon-on-insulator waveguides. Opt. Expr. 2010, 18, 7994–7999. [Google Scholar]
- Xu, Q.; Schmidt, B.; Pradhan, S.; Lipson, M. Micrometre-scale silicon electro-optic modulator. Nature 2005, 435, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Birner, A.; Wehrspohn, R.B.; Gösele, U.M.; Busch, K. Silicon-based photonic crystals. Adv. Mater. 2001, 13, 377–388. [Google Scholar] [CrossRef]
- Ouskova, E.; Sio, L.D.; Vergara, R.; White, T.J.; Tabiryan, N.; Bunning, T.J. Ultra-fast solid state electro-optical modulator based on liquid crystal polymer and liquid crystal composites. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Lavrentovich, M.D.; Sergan, T.A.; Kelly, J.R. Switchable broadband achromatic half-wave plate with nematic liquid crystals. Opt. Lett. 2004, 29, 1411–1413. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, G.; Liu, F.; Kityk, I.; Liu, X.; Zhen, Z. Recent advances in polymer electro-optic modulators. RSC Adv. 2015, 5, 15784–15794. [Google Scholar] [CrossRef]
- Goodson, T.; Gong, S.; Wang, C. Nonlinear-optical suseptibility of a model guest-host polymeric system as investigated by electrooptics and 2nd-harmonic generation. Macromolecules 1994, 27, 4278–4283. [Google Scholar] [CrossRef]
- Schildkraut, J.S. Determination of the electrooptic coefficient of a poled polymer film. Appl. Opt. 1990, 29, 2839–2841. [Google Scholar] [CrossRef] [PubMed]
- Benight, S.J.; Bale, D.H.; Olbricht, B.C.; Dalton, L.R. Organic electro-optics: Understanding material structure/function relationships and device fabrication issues. J. Mater. Chem. 2009, 19, 7466–7475. [Google Scholar] [CrossRef]
- Enami, Y.; Meredith, G.; Peyghambarian, N.; Jen, A.Y. Hybrid electro-optic polymer/sol–gel waveguide modulator fabricated by all-wet etching process. Appl. Phys. Lett. 2003, 83, 4692–4694. [Google Scholar] [CrossRef]
- Derose, C.T.; Himmelhuber, R.; Mathine, D.; Norwood, R.; Luo, J.; Jen, A.Y.; Peyghambarian, N. High Δn strip-loaded electro-optic polymer waveguide modulator with low insertion loss. Opt. Expr. 2009, 17, 3316–3321. [Google Scholar] [CrossRef]
- Bortnik, B.; Hung, Y.C.; Tazawa, H.; Seo, B.J.; Luo, J.; Jen, A.K.Y.; Steier, W.H.; Fetterman, H.R. Electrooptic polymer ring resonator modulation up to 165 GHz. IEEE J. Sel. Top. Quantum Electron. 2007, 13, 104–110. [Google Scholar] [CrossRef]
- Cai, Y.; Jen, A.Y. Thermally stable poled polyquinoline thin film with very large electro-optic response. Appl. Phys. Lett. 1995, 67, 299–301. [Google Scholar] [CrossRef]
- Alloatti, L.; Korn, D.; Palmer, R.; Hillerkuss, D.; Li, J.; Barklund, A.; Dinu, R.; Wieland, J.; Fournier, M.; Fedeli, J.; et al. 42.7 Gbit/s electro-optic modulator in silicon technology. Opt. Expr. 2011, 19, 11841–11851. [Google Scholar]
- Baehr-Jones, T.; Hochberg, M.; Wang, G.; Lawson, R.; Liao, Y.; Sullivan, P.; Dalton, L.; Jen, A.; Scherer, A. Optical modulation and detection in slotted silicon waveguides. Opt. Expr. 2005, 13, 5216–5226. [Google Scholar] [CrossRef]
- Baehr-Jones, T.; Penkov, B.; Huang, J.; Sullivan, P.; Davies, J.; Takayesu, J.; Luo, J.; Kim, T.D.; Dalton, L.; Jen, A.; et al. Nonlinear polymer-clad silicon slot waveguide modulator with a half wave voltage of 0.25 V. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Wang, X.; Lin, C.Y.; Chakravarty, S.; Luo, J.; Jen, A.K.Y.; Chen, R.T. Effective in-device r33 of 735 pm/V on electro-optic polymer infiltrated silicon photonic crystal slot waveguides. Opt. Lett. 2011, 36, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Enami, Y.; Mathine, D.; DeRose, C.; Norwood, R.; Luo, J.; Jen, A.Y.; Peyghambarian, N. Hybrid cross-linkable polymer/sol-gel waveguide modulators with 0.65 V half wave voltage at 1550 nm. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef]
- Enami, Y.; Meredith, G.; Peyghambarian, N.; Kawazu, M.; Jen, A.Y. Hybrid electro-optic polymer and selectively buried sol-gel waveguides. Appl. Phys. Lett. 2003, 82, 490–492. [Google Scholar] [CrossRef]
- Enami, Y.; Derose, C.T.; Mathine, D.; Loychik, C.; Greenlee, C.; Norwood, R.A.; Kim, T.D.; Luo, J.; Tian, Y.; Jen, A.K.Y.; et al. Hybrid polymer/sol-gel waveguide modulators with exceptionally large electro-optic coefficients. Nature Photon. 2007, 1, 180–185. [Google Scholar] [CrossRef]
- Buestrich, R.; Kahlenberg, F.; Popall, M.; Martin, A.; Rösch, O. Low Si-OH ORMOCER®s for dielectrical and optical interconnection technology. In Proceedings of MRS Symposium CC-Hybrid Organic/Inorganic Materials, San Francisco, CA, USA, April 24–28, 2000.
- Groh, W. Overtone absorption in macromolecules for polymer optical fibers. Die Makromol. Chem. 1988, 189, 2861–2874. [Google Scholar] [CrossRef]
- Kahlenberg, F.; Popall, M. ORMOCER®s (Organic-inorganic hybrid polymers) for telecom applications: structure/property correlations. In Proceedings of MRS Symposium EE-Organic/Inorganic Hybrid Materials, Boston, MA, USA, 29 November–3 December 2004; Volume 847, pp. 419–430.
- Oubaha, M.; Smaıhi, M.; Etienne, P.; Coudray, P.; Moreau, Y. Spectroscopic characterization of intrinsic losses in an organic-inorganic hybrid waveguide synthesized by the sol-gel process. J. Non-Crystalline Sol. 2003, 318, 305–313. [Google Scholar] [CrossRef]
- Himmelhuber, R.; DeRose, C.C.; Norwood, R.A.; Peyghambarian, N. UV-patternable inorganic-organic hybrid materials tailored for use in electro-optic modulators. In Proceedings of SPIE the Linear and Nonlinear Optics of Organic Materials, San Diego, CA, USA, 2 September 2008.
- Soppera, O.; Moreira, P.; Leite, A.; Marques, P. Low-loss Photopatternable Hybrid Sol-Gel Materials. J. Sol-Gel Sci. Technol. 2005, 35, 27–39. [Google Scholar] [CrossRef]
- Soppera, O.; Moreira, P.; Marques, P.; Leite, A. Influence of temperature and environment humidity on the transmission spectrum of sol-gel hybrid channel waveguides. Opt. Commun. 2007, 271, 430–435. [Google Scholar] [CrossRef]
- Kahlenberg, F. Structure-Property Correlations in Fluoroaryl Functionalized Inorganic-Organic Hybrid Polymers for Telecom Applications. Ph.D. Thesis, Julius-Maximilians-Universität Würzburg, Bayern, Germany, 2004. [Google Scholar]
- Varma, I.K.; Tomar, A.K.; Anand, R.C. Copolymerization of γ-methacryloxy propyl trimethoxy silane and methyl methacrylate. J. Appl. Polym. Sci. 1987, 33, 1377–1388. [Google Scholar] [CrossRef]
- Miller, J.D.; Ishida, H. Quantitative intermolecular reaction of hydrolyzed trialkoxysilanes at submonolayer, monolayer, and multilayer surface coverages. Langmuir 1986, 2, 127–131. [Google Scholar] [CrossRef]
- Jeong, S.; Moon, J. Fabrication of inorganic-organic hybrid films for optical waveguide. J. Non-Crystalline Sol. 2005, 351, 3530–3535. [Google Scholar] [CrossRef]
- Groh, W.; Zimmermann, A. What is the lowest refractive index of an organic polymer? Macromolecules 1991, 24, 6660–6663. [Google Scholar] [CrossRef]
- Kahlenberg, F.; Buestrich, R.; Popall, M. Controlled synthesis of perfluoroaryl functionalized hybrid materials for optical applications based on NMR spectroscopy and molecular modeling. In Proceedings of MRS Symposium: Organic/Inorganic Hybrid Materials, San Francisco, CA, USA, 24–28 April 2000.
- Amao, Y. Probes and Polymers for Optical Sensing of Oxygen. Microchimica. Acta. 2003, 143, 1–12. [Google Scholar] [CrossRef]
- Lee, T.; Guymon, C.; Jönsson, E.S.; Hoyle, C. The effect of monomer structure on oxygen inhibition of (meth)acrylates photopolymerization. Polymer 2004, 45, 6155–6162. [Google Scholar] [CrossRef]
- Oubaha, M.; Etienne, P.; Calas, S.; Sempere, R.; Nedelec, J.; Moreau, Y. Spectroscopic characterization of sol-gel organo-siloxane materials synthesized from aliphatic and aromatic alcoxysilanes. J. Non-Crystalline Sol. 2005, 351, 2122–2128. [Google Scholar] [CrossRef]
- Fardad, A.; Andrews, M.; Milova, G.; Malek-Tabrizi, A.; Najafi, I. Fabrication of ridge waveguides: A new solgel route. Appl. Opt. 1998, 37, 2429–2434. [Google Scholar] [CrossRef] [PubMed]
- Graziola, F.; Girardi, F.; Bauer, M.; Maggio, R.D.; Rovezzi, M.; Bertagnolli, H.; Sada, C.; Rossetto, G.; Gross, S. UV-photopolymerisation of poly(methyl methacrylate)-based inorganic-organic hybrid coatings and bulk samples reinforced with methacrylate-modified zirconium oxocluster. Polymer 2008, 49, 4332–4343. [Google Scholar] [CrossRef]
- Soppera, O.; Croutxé-Barghorn, C.; Carré, C. Optimization of radical photopolymerization in hybrid sol-gel glasses: Advantages of bicomponent photoactive systems. In Proceedings of MRS Symposium: Organic-Inorganic Materials, San Francisco, CA, USA, 1–5 April, 2002; Volume 726, pp. 303–310.
- Arkles, B.; Larson, G. Silanes and Silicones, Gelest Catalog 3000—A; Gelest Inc.: Morrisville, PA, USA, 2004. [Google Scholar]
- DeRose, C. Electro-optic Polymers: Material and Devices. Ph.D. Thesis, University of Arizona, Tuson, AZ, USA,, 2009. [Google Scholar]
- Sprave, M.; Blum, R.; Eich, M. High electric field conduction mechanisms in electrode poling of electro-optic polymers. Appl. Phys. Lett. 1996, 69, 2962–2964. [Google Scholar] [CrossRef]
- DeRose, C.T.; Enami, Y.; Loychik, C.; Norwood, R.A.; Mathine, D.; Fallahi, M.; Peyghambarian, N.; Luo, J.D.; Jen, A.K.Y.; Kathaperumal, M.; et al. Pockel’s coefficient enhancement of poled electro-optic polymers with a hybrid organic-inorganic sol-gel cladding layer. Appl. Phys. Lett. 2006, 89, 131102. [Google Scholar] [CrossRef]
- Drummond, J.P.; Clarson, S.J.; Zetts, J.S.; Hopkins, F.K.; Caracci, S.J. Enhanced electro-optic poling in guest-host systems using conductive polymer-based cladding layers. Appl. Phys. Lett. 1999, 74, 368–370. [Google Scholar] [CrossRef]
- Huddleston, J.B.; Grote, J.G.; Nelson, R.L.; Zetts, J.S.; Hopkins, F.K. Maximizing poling efficiency of a 3-layer NLO polymer-based structure using conductive polymer cladding layers. Proc. SPIE 2001, 4290. [Google Scholar] [CrossRef]
- Grote, J.G.; Zetts, J.S.; Nelson, R.L.; Hopkins, F.K.; Zhang, C.H.; Dalton, L.R.; Steier, W.H. Conductive cladding layers for electrode-poled nonlinear optic polymer electro-optics. Proc. SPIE 2000, 4114. [Google Scholar] [CrossRef]
- Enami, Y.; Jouane, Y.; Luo, J.; Jen, A.K. Enhanced conductivity of sol-gel silica cladding for efficient poling in electro-optic polymer/TiO2 vertical slot waveguide modulators. Opt. Expr. 2014, 22, 30191–30199. [Google Scholar] [CrossRef] [PubMed]
- Himmelhuber, R. Sol-Gel Materials for Optical Waveguide applications. Ph.D. Thesis, The University of Arizona, Tuson, AZ, USA, June 2014. [Google Scholar]
- Vijay, V.; Rao, A.D.; Narayan, K.S. In situ studies of strain dependent transport properties of conducting polymers on elastomeric substrates. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Kreuer, K. Proton conductivity: Materials and applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A. Synthesis and characterization of proton conducting polymer membranes for fuel cells. J. Membr. Sci. 2003, 225, 63–76. [Google Scholar] [CrossRef]
- Huang, S.; Kim, T.D.; Luo, J.; Hau, S.K.; Shi, Z.; Zhou, X.H.; Yip, H.L.; Jen, A.K.Y. Highly efficient electro-optic polymers through improved poling using a thin TiO2-modified transparent electrode. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Enami, Y.; Yuan, B.; Tanaka, M.; Luo, J.; Jen, A.Y. Electro-optic polymer/TiO2 multilayer slot waveguide modulators. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Enami, Y.; Kayaba, Y.; Luo, J.; Jen, A.K.Y. Mesoporous sol-gel silica cladding for hybrid TiO2/electro-optic polymer waveguide modulators. Opt. Expr. 2014, 22, 16418–16423. [Google Scholar] [CrossRef] [PubMed]
- The SEO Materials are Commercially. Available online: www.soluxra.com (accessed on 15 April 2015).
- Enami, Y.; Luo, J.; Jen, A.K.Y. Short hybrid polymer/sol-gel silica waveguide switches with high in-device electro-optic coefficient based on photostable chromophore. AIP Adv. 2011, 1. [Google Scholar] [CrossRef]
- Jouane, Y.; Chang, Y.; Zhang, D.; Luo, J.; Jen, A.; Enami, Y. Unprecedented highest electro-optic coefficient of 226 pm/V for electro-optic polymer/TiO2 multilayer slot waveguide modulators. Opt. Expr. 2014, 22, 27725–27732. [Google Scholar] [CrossRef] [PubMed]
- Kajzar, F.; Swalen, J. Organic Thin Films for Waveguiding Nonlinear Optics; Gordon and Breach Publishers: Amsterdam, The Netherlands, 1996. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Himmelhuber, R.; Norwood, R.A.; Enami, Y.; Peyghambarian, N. Sol-Gel Material-Enabled Electro-Optic Polymer Modulators. Sensors 2015, 15, 18239-18255. https://doi.org/10.3390/s150818239
Himmelhuber R, Norwood RA, Enami Y, Peyghambarian N. Sol-Gel Material-Enabled Electro-Optic Polymer Modulators. Sensors. 2015; 15(8):18239-18255. https://doi.org/10.3390/s150818239
Chicago/Turabian StyleHimmelhuber, Roland, Robert A. Norwood, Yasufumi Enami, and Nasser Peyghambarian. 2015. "Sol-Gel Material-Enabled Electro-Optic Polymer Modulators" Sensors 15, no. 8: 18239-18255. https://doi.org/10.3390/s150818239





