Investigation of the Performance of HEMT-Based NO, NO2 and NH3 Exhaust Gas Sensors for Automotive Antipollution Systems
Abstract
:1. Introduction
2. Experimental Section
2.1. Operating Principles of AlGaN/GaN HEMT-Based Gas Sensors
2.2. Description of the Gas Sensor Fabricated and the Test Method Used
3. Results and Discussion
3.1. Performance Enhancement of a Pt-AlGaN/GaN HEMT-Based Sensor Using an Optimized Design
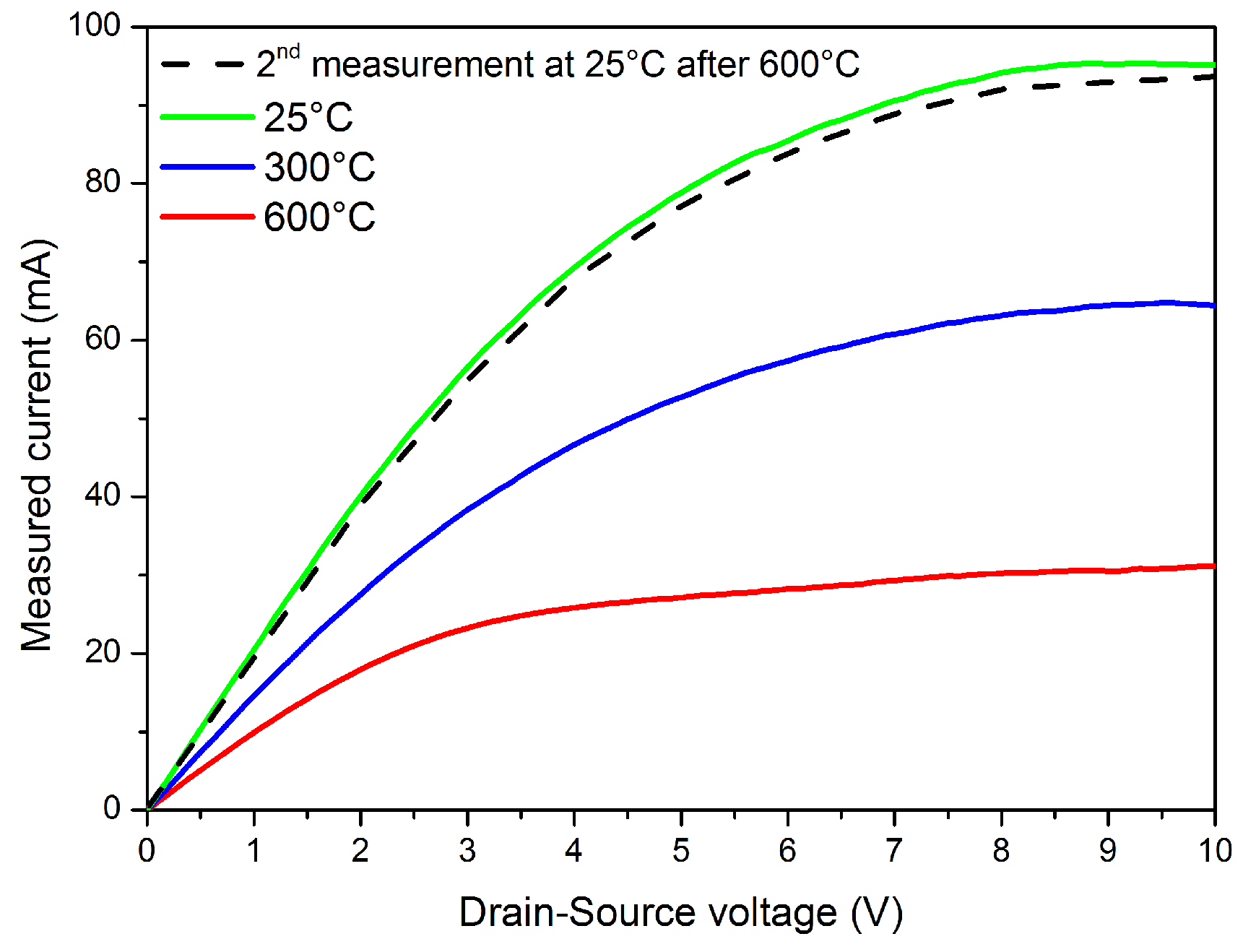
3.2. Performances of the Pt-AlGaN/GaN HEMT-Based Sensor at an Operation Temperature between 300 °C and 600 °C
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, S.; Chaudhuir, S.N.; Dutta, D. NOx reduction by using UREA injection and marine ferromanganese nodule as SCR of a Diesel engine fulled with pongamia pinnata methyl ester. Int. J. Mod. Eng. Res 2012, 3, 779–784. [Google Scholar]
- Kato, N.; Nakagaki, K.; Ina, N. Thick Film ZrO2 NOx Sensor; SAE Technical Paper 960334; NGK Insulators, Ltd.: Aichi Prefecture, Japan, 1996. [Google Scholar]
- Kunimoto, A.; Hasei, M.; Yan, Y.; Gao, Y.; Ono, T.; Nakanouchi, Y. New Total-NOx Sensor Based on Mixed Potential for Automobiles; SAE Technical Paper 1999-01-1280; NGK Insulators, Ltd.: Aichi Prefecture, Japan, 1999. [Google Scholar]
- Kunimoto, A.; Hasei, M.; Yan, Y.; Gao, Y.; Ono, T.; Nakanouchi, Y. New Total-NOx Sensor Based on Mixed Potential for Automobiles. SAE Tech. Pap. 1999. [Google Scholar] [CrossRef]
- Chen, H.-I.; Chou, P.-C.; Liou, J.-K.; Chen, C.-C.; Chang, C.-F.; Liu, W.-C. Hydrogen-Sensing Properties of a Pd/AlGaN/GaN-Based Field-Effect Transistor under a Nitrogen Ambience. IEEE Sens. J. 2013, 13, 1787–1793. [Google Scholar]
- Chen, H.-I.; Hsu, C.-S.; Huang, C.-C.; Chang, C.-F.; Chou, P.-C.; Liu, W.-C. On an Ammonia Gas Sensor Based on a Pt/AlGaN Heterostructure Field-Effect Transistor. IEEE Electron. Device Lett. 2012, 33, 612–614. [Google Scholar] [CrossRef]
- Makoto, M.; Shu, F.; Takashi, E. Demonstration of NOx gas sensing for Pd/ZnO/GaN heterojunction diodes. J. Vac. Sci. Technol. 2015, 33. [Google Scholar] [CrossRef]
- Benard, S.; Retailleau, L.; Gaillard, F.; Vernoux, P.; Giroir-Fendler, A. Supported platinum catalysts for nitrogen oxide sensors. Appl. Catal. B 2005, 55, 11–12. [Google Scholar] [CrossRef]
- Song, J.; Lu, W.; Flynn, J.; Brandes, G. Pt-AlGaN/GaN Schottky diodes operated at 800 °C for hydrogen sensing. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Song, J.; Lu, W.; Flynn, J.; Brandes, G. AlGaNGaN Schottky diode hydrogen sensor performance at high temperature with different catalytic metals. Solid-State Electron. 2005, 49, 1330–1334. [Google Scholar] [CrossRef]
- Rygera, I.; Vanko, G.; Kunzo, P.; Lalinsky, T.; Vallo, M.; Plecenik, A.; Satrapinsky, L.; Plecenik, T. AlGaN/GaN HEMT Based Hydrogen Sensors with Gate Absorption Layers Formed by High Temperature Oxidation. Procedia Eng. 2012, 47, 518–521. [Google Scholar] [CrossRef]
- Hung, S.; Chang, C.; Hsu, C.; Chu, B.; Lo Fong, C.; Hsu, C.; Pearton, S.; Holzworth, M.; Whiting, P.; Rudawski, N.; et al. SnO2 functionalized AlGaN/GaN high electron mobility transistor for hydrogen sensing applications. Int. J. Hydrog. Energy 2012, 37, 13783–13788. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Tilak, V.; Matocha, K.; Sandvik, P. Novel GaN and SiC based gas sensors. Phys. Status Solidi 2006, 3, 548–553. [Google Scholar] [CrossRef]
- Tilak, V.; Matocha, K.; Sandvik, P. Pt/GaN Schottky diodes for harsh environment NO sensing applications. Phys. Status Solidi 2005, 7, 2555–2558. [Google Scholar] [CrossRef]
- Bishop, C.; Salvestrini, J.P.; Halfaya, Y.; Sundaram, S.; el Gmili, Y.; Pradere, L.; Marteau, J.Y.; Assouar, M.B.; Voss, P.L.; Ougazzaden, A. Highly sensitive detection of NO2 gas using BGaN/GaN superlattice-based double Schottky junction sensors. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef]
- Schalwig, J.; Muller, G.; Eickhoff, M.; Ambacher, O.; Stutzmann, M. Gas sensitive GaN/AlGaN-heterostructures. Sens. Actuators B Chem. 2002, 87, 425–430. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y. 2-D analytical model for current-voltage characteristics and transconductance of AlGaN/GaN MODFETs. IEEE Electron. Dev. 2008, 55, 261–267. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Wu, X.-H.; Su, Q.; Li, Y.-F.; Zhou, Z.-L. Ammonia sensing characteristics of Pt and SiO2 doped SnO2. Solid-State Electron. 2001, 45, 347–350. [Google Scholar] [CrossRef]
- Wang, Y.D.; Wu, X.H.; Su, Q.; Li, Y.F.; Zhou, Z.L. Ammonia-sensing characteristics of Pt and SiO2 doped SnO2 materials. Solid-State Electron. 2001, 45, 347–350. [Google Scholar] [CrossRef]
- Schalwig, J.; Muller, G.; Eickhoff, M.; Ambacher, O.; Stutzmann, M. Group III-nitride-based gas sensors for combustion monitoring. Mater. Sci. Eng. B 2002, 93, 207–214. [Google Scholar] [CrossRef]
- Wingbrant, H.; Svenningstorp, H.; Salomonsson, P.; Kubinski, D.; Visser, J.H.; Löfdahl, M.; Spetz, A.L. Using a MISiC-FET Sensor for Detecting NH3 in SCR Systems. IEEE Sens. J. 2005, 5, 1099–1105. [Google Scholar] [CrossRef]
- Lundstrom, I.; Sundgren, H.; Winquist, F.; Eriksson, M.; Krantz-Rulcker, C.; Lloyd-Spetz, A. Twenty-five years of field effect gas sensor research in LinkÃűping. Sens. Actuators B Chem. 2007, 121, 247–262. [Google Scholar] [CrossRef]
- Chen, H.-I.; Liu, Y.-J.; Huang, C.-C.; Hsu, C.-S.; Chang, C.-F.; Liu, W.-C. Ammonia sensing characteristics of a Pt-AlGaN/GaN Schottky diode. Sens. Actuators B Chem. 2011, 45, 347–350. [Google Scholar] [CrossRef]
- Hussein, A.S.; Ghazai, A.J.; Salman, E.A.; Hassan, Z. Effects of traps and polarization charges on device performance of AlGaN/GaN high electron mobility transistors. Superlattices Microstruct. 2013, 63, 141–148. [Google Scholar] [CrossRef]
- Chang, S.J.; Wei, S.C.; Su, Y.K.; Liu, C.H.; Chen, S.C.; Liaw, U.H.; Hsu, T.H. AlGaN/GaN modulation-doped field-effect transistors with an Mg-doped carrier confinement layer. Jpn. J. Appl. Phys. 2003, 42, 3316–3319. [Google Scholar] [CrossRef]








| Type of Gas | Concentration (ppm) | Temperature (C) | I0 (mA) | Delta I (mA) | Sensitivity (%) | Response Time (min) |
|---|---|---|---|---|---|---|
| NO | 900 | 300 | 54.5 | 7 | 12.8 | 1.7 |
| 600 | 12.5 | 3 | 24 | 0.43 | ||
| NO2 | 900 | 300 | 44 | 14 | 33 | 27 |
| 600 | 13 | 5 | 38.5 | 1.2 | ||
| NH3 | 15 | 300 | 49.5 | 7 | 13 | 4.3 |
| 600 | 12 | 4 | 33 | 0.45 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halfaya, Y.; Bishop, C.; Soltani, A.; Sundaram, S.; Aubry, V.; Voss, P.L.; Salvestrini, J.-P.; Ougazzaden, A. Investigation of the Performance of HEMT-Based NO, NO2 and NH3 Exhaust Gas Sensors for Automotive Antipollution Systems. Sensors 2016, 16, 273. https://doi.org/10.3390/s16030273
Halfaya Y, Bishop C, Soltani A, Sundaram S, Aubry V, Voss PL, Salvestrini J-P, Ougazzaden A. Investigation of the Performance of HEMT-Based NO, NO2 and NH3 Exhaust Gas Sensors for Automotive Antipollution Systems. Sensors. 2016; 16(3):273. https://doi.org/10.3390/s16030273
Chicago/Turabian StyleHalfaya, Yacine, Chris Bishop, Ali Soltani, Suresh Sundaram, Vincent Aubry, Paul L. Voss, Jean-Paul Salvestrini, and Abdallah Ougazzaden. 2016. "Investigation of the Performance of HEMT-Based NO, NO2 and NH3 Exhaust Gas Sensors for Automotive Antipollution Systems" Sensors 16, no. 3: 273. https://doi.org/10.3390/s16030273





