Methods and Best Practice to Intercompare Dissolved Oxygen Sensors and Fluorometers/Turbidimeters for Oceanographic Applications
Abstract
:1. Introduction
2. Materials and Methods
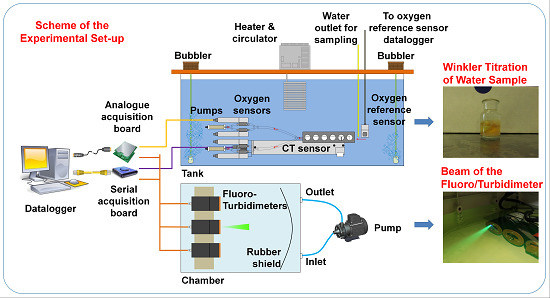
2.1. Hardware and Software Tools for the Intercomparison of Dissolved Oxygen Sensors
2.2. IntercomparisonProtocol for Dissolved Oxygen Sensors
2.3. Hardware and Software Tools for the Intercomparison of Chlorophyll-a and Turbidity Sensors
2.4. IntercomparisonProtocol for Fluorometers/Turbidimeters
3. Results
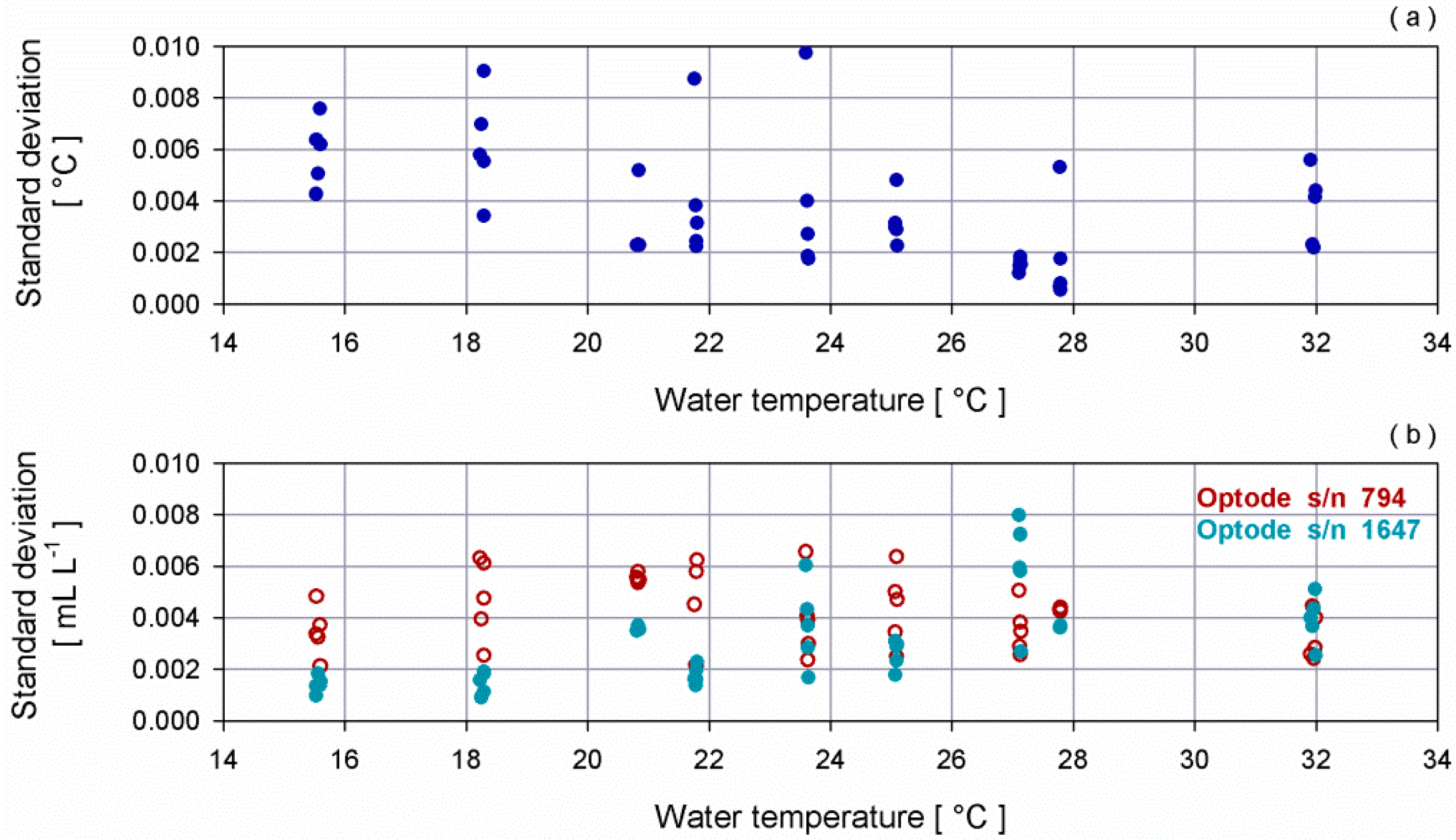
3.1. Laboratory Tests for Dissolved Oxygen Sensors
3.2. Laboratory Tests for Chlorophyll-a Sensors
3.3. Laboratory Tests for Turbidity Sensors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A: Dissolved Oxygen Sensors Technology
Appendix B: Chlorophyll-a and Turbidity Sensors Technology
References
- Lampitt, R.; Favali, P.; Barnes, C.R.; Church, M.J.; Cronin, M.F.; Hill, K.L.; Kaneda, Y.; Karl, D.M.; Knap, A.H.; McPhaden, M.J.; et al. In situ Sustained Eulerian Observatories. In Proceedings of the OceanObs’09: Sustained Ocean Observations and Information for Society, Venice, Italy, 21–25 September 2009.
- Garzoli, S.; Boebel, O.; Bryden, H.; Fine, R.; Fukasawa, M.; Gladyshev, S.; Johnson, G.; Johnson, M.; MacDonald, A.; Meinen, C.; et al. Progressing Towards Global Sustained Deep Ocean Observations. In Proceedings of OceanObs’09: Sustained Ocean Observations and Information for Society, Venice, Italy, 21–25 September 2009.
- Heimbürger, L.E.; Lavigne, H.; Migon, C.; D’Ortenzio, F.; Estournel, C.; Coppola, L.; Miquel, J.C. Temporal variability of vertical export flux at the DYFAMED time-series station (Northwestern Mediterranean Sea). Prog. Oceanogr. 2013, 119, 59–67. [Google Scholar] [CrossRef]
- Dickey, T.; Zedler, S.; Yu, X.; Doney, S.C.; Frye, D.; Jannasch, H.; Manov, D.; Sigurdson, D.; McNeil, J.D.; Dobeck, L.; et al. Physical and biogeochemical variability from hours to years at the Bermuda Testbed Mooring site: June 1994–March 1998. Deep Sea Res. II Top. Stud. Oceanogr. 2001, 48, 2105–2140. [Google Scholar] [CrossRef]
- García-Reyes, M.; Largier, J.L.; Sydeman, W.J. Synoptic-scale upwelling indices and predictions of phyto- and zooplankton populations. Prog. Oceanogr. 2014, 120, 177–188. [Google Scholar] [CrossRef]
- Hoteit, I.; Triantafyllou, G.; Petihakis, G. Towards a data assimilation system for the Cretan Sea ecosystem using a simplified Kalman filter. J. Mar. Syst. 2004, 45, 159–171. [Google Scholar]
- Triantafyllou, G.; Petihakis, G.; Allen, I.J. Assessing the performance of the Cretan Sea ecosystem model with the use of high frequency M3A buoy data set. Ann. Geophys. 2003, 21, 365–375. [Google Scholar] [CrossRef]
- Marty, J.C.; Chiavérini, J. Seasonal and interannual variations in phytoplankton production at DYFAMED time-series station, northwestern Mediterranean Sea. Deep Sea Res. II Top. Stud. Oceanogr. 2002, 49, 2017–2030. [Google Scholar] [CrossRef]
- Faugeras, B.; Lévy, M.; Mémery, L.; Verron, J.; Blum, J.; Charpentier, I. Can biogeochemical fluxes be recovered from nitrate and chlorophyll data? A case study assimilating data in the Northwestern Mediterranean Sea at the JGOFS-DYFAMED station. J. Mar. Syst. 2003, 40–41, 99–125. [Google Scholar] [CrossRef]
- De Fommervault, O.P.; Migon, C.; D’Ortenzio, F.; d’Alcalà, M.R.; Coppola, L. Temporal variability of nutrient concentrations in the northwestern Mediterranean sea (DYFAMED time-series station). Deep Sea Res. I Oceanogr. Res. Pap. 2015, 100, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Drakopoulos, P.; Petihakis, G.; Valavanis, V.; Nittis, K.; Triantafyllou, G. Optical variability associated with phytoplankton dynamics in the Cretan Sea during 2000 and 2001. In Building the European Capacity in Operational Oceanography; Elsevier B.V.: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Cianca, A.; Santana, R.; Hartman, S.E.; Martín-González, J.M.; González-Dávila, M.; Rueda, M.J.; Llinás, O.; Neuer, S. Oxygen dynamics in the North Atlantic subtropical gyre. Deep Sea Res. II Top. Stud. Oceanogr. 2013, 93, 135–147. [Google Scholar] [CrossRef]
- Lindstrom, E.; Gunn, J.; Fischer, A.; McCurdy, A.; Glover, L.K.; Members, T.T. A Framework for Ocean Observing; UNESCO: Paris, France, 2012. [Google Scholar]
- National Research Council. Enabling Ocean Research in the 21st Century: Implementation of a Network of Ocean Observatories; The National Academic Press: Washington, DC, USA, 2003. [Google Scholar]
- Cronin, M.F.; Weller, R.A.; Lampitt, R.S.; Send, U. Ocean reference stations. In Earth Observation; Rustamov, R.B., Salahova, S.E., Eds.; InTech: Rijeka, Croatia, 2002; pp. 203–228. [Google Scholar]
- Huang, Y.; Schmitt, F.G. Time dependent intrinsic correlation analysis of temperature and dissolved oxygen time series using empirical mode decomposition. J. Mar. Syst. 2014, 130, 90–100. [Google Scholar] [CrossRef]
- Kuss, J.; Roeder, W.; Wlost, K.P.; DeGrandpre, M.D. Time-series of surface water CO2 and oxygen measurements on a platform in the central Arkona Sea (Baltic Sea): Seasonality of uptake and release. Mar. Chem. 2006, 101, 220–232. [Google Scholar] [CrossRef]
- Martini, S.; Nerini, D.; Tamburini, C. Relation between deep bioluminescence and oceanographic variables: A statistical analysis using time-frequency decompositions. Prog. Oceanogr. 2014, 127, 117–128. [Google Scholar] [CrossRef]
- Bergondo, D.L.; Kester, D.R.; Stoffel, H.E.; Woods, W.L. Time-series observations during the low sub-surface oxygen events in Narragansett Bay during summer 2001. Mar. Chem. 2005, 97, 90–103. [Google Scholar] [CrossRef]
- Waniek, J.J.; Schulz-Bull, D.E.; Kuss, J.; Blanz, T. Long time series of deep water particle flux in three biogeochemical provinces of the northeast Atlantic. J. Mar. Syst. 2005, 56, 391–415. [Google Scholar] [CrossRef]
- Martini, M.; Butman, B.; Mickelson, M. Long-Term Performance of AanderaaOptodes and Sea-Bird SBE-43 Dissolved-Oxygen Sensors Bottom Mounted at 32 m in Massachusetts Bay. J. Atmos. Ocean. Technol. 2007, 24, 1924–1935. [Google Scholar] [CrossRef]
- Bittig, H.C.; Fiedler, B.; Steinhoff, T.; Körtzinger, A. A novel electrochemical calibration setup for oxygen sensors and its use for the stability assessment of Aanderaa optodes. Limnol. Oceanogr. Meth. 2012, 10, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Gruber, N.; Doney, S.C.; Emerson, S.R.; Gilbert, D.; Kobayashi, T.; Körtzinger, A.; Johnson, G.C.; Johnson, K.S.; Riser, S.C.; Ulloa, O. Adding oxygen to argo: Developing a global in situ observatory for ocean deoxygenation and biogeochemistry. In Proceedings of the OceanObs’09: Sustained Ocean Observations and Information for Society, Venice, Italy, 21–25 September 2009.
- Takeshita, Y.; Martz, T.R.; Johnson, K.S.; Plant, J.N.; Gilbert, D.; Riser, S.C.; Neill, C.; Tilbrook, B. A climatology-based quality control procedure for profiling float oxygen data. J. Geophys. Res. Oceans 2013, 118, 5640–5650. [Google Scholar] [CrossRef]
- Murray, A.P.; Gibbs, C.F.; Longmore, A.R.; Flett, D.J. Determination of chlorophyll in marine waters: Intercomparison of a rapid HPLC method with full HPLC, spectrophotometric and fluorometric methods. Mar. Chem. 1986, 19, 211–227. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Dickson, A.G. Determination of dissolved oxygen in seawater by Winkler titration. In WOCE Operations Manual; World Ocean Circulation Experiment: Woods Hole, MC, USA, 1996. [Google Scholar]
- Carpenter, J.H. The Chesapeake Bay Institute technique for the Winkler dissolved oxygen method. Limnol. Oceanogr. 1965, 10, 141–143. [Google Scholar] [CrossRef]
- Carpenter, J.H. The accuracy of the Winkler method for dissolved oxygen. Limnol. Oceanogr. 1965, 10, 135–140. [Google Scholar] [CrossRef]
- Carpenter, J.H. New measurements of oxygen solubility in pure and natural waters. Limnol. Oceanogr. 1966, 11, 264–277. [Google Scholar] [CrossRef]
- Murray, C.N.; Riley, J.P. The solubility of gases in distilled water and sea water—II. Oxygen. Deep-Sea Res. Oceanogr. Abstr. 1969, 16, 311–320. [Google Scholar] [CrossRef]
- Coppola, L.; Salvetat, F.; Delauney, L.; Machoczek, D.; Karstensen, J.; Sparnocchia, S.; Thierry, V.; Hydes, D.; Haller, M.; Nair, R.; et al. White Paper on Dissolved Oxygen Measurements: Scientific Needs and Sensors Accuracy; Jerico Project; Ifremer: Brest, France, 2013. [Google Scholar]
- Owens, W.B.; Millard, R.C., Jr. A New Algorithm for CTD Oxygen Calibration. J. Phys. Oceanogr. 1985, 15, 621–631. [Google Scholar] [CrossRef]
- Joos, F.; Plattner, G.K.; Stocker, T.F.; Körtzinger, A.; Wallace, D.W.R. Trends in marine dissolved oxygen: Implications for ocean circulation changes and the carbon budget. EOS Trans. Am. Geophys. Union 2003, 84, 197–201. [Google Scholar] [CrossRef]
- Bittig, H.C.; Fiedler, B.; Scholz, R.; Krahmann, G.; Körtzinger, A. Time response of oxygen optodes on profiling platforms and its dependence on flow speed and temperature. Limnol. Oceanogr. Meth. 2014, 12, 617–636. [Google Scholar] [CrossRef]
- Hongve, D.; Kesson, G. Comparison of nephelometric turbidity measurements using wavelengths 400–600 and 860 nm. Water Res. 1998, 32, 3143–3145. [Google Scholar] [CrossRef]
- Downing, J. Turbidity Monitoring. In Environmental Instrumentation and Analysis Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Carrol, M.; Chigounis, D.; Gilbert, S.; Gundersen, K.; Hayashi, K.; Janzen, C.; Johengen, T.; Koles, T.; Laurier, F.; McKissack, T.; et al. Performance Verification Statement for the Wet Labs ECO FLNTUSB Fluorometer; Alliance for Coastal Technologies: Solomons, MD, USA, 2006. [Google Scholar]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- D’Asaro, E.A.; McNeil, C. Calibration and Stability of Oxygen Sensors on Autonomous Floats. J. Atmos. Ocean. Technol. 2013, 30, 1896–1906. [Google Scholar] [CrossRef]
- Wanninkhof, R.; Asher, W.E.; Ho, D.T.; Sweeney, C.; McGillis, W.R. Advances in Quantifying Air-Sea Gas Exchange and Environmental Forcing. Annu. Rev. Mar. Sci. 2009. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.J.; Davis, F. The blank can make a big difference in oceanographic measurements. Limnol. Oceanogr. Bull. 2003, 12, 29–35. [Google Scholar]
- Goodin, D.G.; Han, L.; Fraser, R.N.; Rundquist, D.C.; Stebbins, W.A. Analysis of suspended solids in water using remotely sensed high resolution derivative spectra. Photogramm. Eng. Remote Sens. 1993, 598, 505–510. [Google Scholar]
- Twardowski, M.S.; Claustre, H.; Freeman, S.A.; Stramski, D.; Huot, Y. Optical backscattering properties of the “clearest” natural waters. Biogeosciences 2007, 4, 1041–1058. [Google Scholar] [CrossRef]
- Earp, A.; Hanson, C.E.; Ralph, P.J.; Brando, V.E.; Allen, S.; Baird, M.; Clementson, L.; Daniel, P.; Dekker, A.G.; Fearns, P.R.C.S.; et al. Review of fluorescent standards for calibration of in situ fluorometers: Recommendations applied in coastal and ocean observing programs. Opt. Express 2011, 19, 26768–26782. [Google Scholar] [CrossRef] [PubMed]
- Diehl, H.; Markuszewski, R. Studies on fluorescein—VII: The fluorescence of fluorescein as a function of pH. Talanta 1989, 36, 416–418. [Google Scholar] [CrossRef]
- Sjöback, R.; Nygren, J.; Kubista, M. Absorption and fluorescence properties of fluorescein. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995, 51, L7–L21. [Google Scholar] [CrossRef]
- Esteves, V.I.; Santos, E.B.H.; Duarte, A.C. Study of the effect of pH, salinity and DOC on fluorescence of synthetic mixtures of freshwater and marine salts. J. Environ. Monit. 1999, 1, 251–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzano, R.; Pensieri, S.; Pensieri, L.; Cardin, V.; Brunetti, F.; Bensi, M.; Petihakis, G.; Tsagaraki, T.M.; Ntoumas, M.; Podaras, D.; et al. The M3A Network of Open Ocean Observatories in the Mediterranean Sea. In Proceedings of the OCEANS 2013 MTS/IEEE, Bergen, Norway, 10–14 June 2013; pp. 1–10.
- Lampitt, R.; Cristina, L. FixO3 Network Project: Integration, Harmonization and Innovation. In Proceedings of the European Geosciences Union General Assembly 2016, Vienna, Austria, 17–22 April 2016.
- Canepa, E.; Pensieri, S.; Bozzano, R.; Faimali, M.; Traverso, P.; Cavaleri, L. The ODAS Italia 1 buoy: More than forty years of activity in the Ligurian Sea. Prog. Oceanogr. 2015, 135, 48–63. [Google Scholar] [CrossRef]
- Edwards, B.; Murphy, D.; Janzen, C.; Larson, N. Calibration, Response, and Hysteresis in Deep-Sea Dissolved Oxygen Measurements. J. Atmos. Ocean. Technol. 2010, 27, 920–931. [Google Scholar] [CrossRef]
- Carlson, J. Development of an optimized dissolved oxygen sensor for oceanographic profiling. Int. Ocean Syst. 2010, 6, 20–21. [Google Scholar]
- Clark, L.C., Jr.; Wolf, R.; Granger, D.; Taylor, Z. Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 1953, 6, 189–193. [Google Scholar] [PubMed]
- Garcia, H.E.; Gordon, L.I. Oxygen solubility in sea water: Better fitting equations. Limnol. Oceanogr. 1992. [Google Scholar] [CrossRef]
- Demas, J.N.; de Graff, B.A.; Coleman, P.B. Oxygen sensors based on luminescence quenching. Anal. Chem. 1999, 71, 793A–800A. [Google Scholar] [CrossRef] [PubMed]
- Klimant, I.; Kühl, M.; Glud, R.N.; Holst, G. Optical measurement of oxygen and temperature in microscale: Strategies and biological applications. Sens. Actuators B Chem. 1997, 38, 29–37. [Google Scholar] [CrossRef]
- Tengberg, A.; Hovdenes, J.; Andersson, H.J.; Brocandel, O.; Diaz, R.; Hebert, D.; Arnerich, T.; Huber, C.; Körtzinger, A.; Khripounoff, A.; et al. Evaluation of a lifetime-based optode to measure oxygen in aquatic systems. Limnol. Oceanogr. Meth. 2006. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, C.J. A method for the continuous measurement of in vivo chlorophyll concentration. Deep Sea Res. Oceanogr. Abstr. 1966, 13, 223–227. [Google Scholar] [CrossRef]
- Boss, E.; Taylor, L.; Gilbert, S.; Gundersen, K.; Hawley, N.; Janzen, C.; Johengen, T.; Purcell, H.; Robertson, C.; Schar, D.W.H.; et al. Comparison of inherent optical properties as a surrogate for particulate matter concentration in coastal waters. Limnol. Oceanogr. Meth. 2009. [Google Scholar] [CrossRef]
- Falkowski, P.; Kiefer, D.A. Chlorophyll-a fluorescence in phytoplankton: Relationship to photosynthesis and biomass. J. Plankton Res. 1985, 7, 715–731. [Google Scholar] [CrossRef]
- Suggett, D.J.; MacIntyre, H.L.; Geider, R.J. Evaluation of biophysical and optical determinations of light absorption by photosystem II in phytoplankton. Limnol. Oceanogr. Meth. 2004. [Google Scholar] [CrossRef]
- Suggett, D.J.; Prasil, O.; Borowitzka, M.A. Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Springer: New York, NY, USA, 2011. [Google Scholar]














| Chlorophyll-a Offset | WET Labs, Inc. ECO-FLNTUS Sensors | |||
|---|---|---|---|---|
| s/n | 2776 | 3372 | 615 | |
| SF·(BLANKTAPE − BLANKAIR) | 0.026 | −0.034 | 0.023 | |
| SF·(BLANKTAPE − BLANKFSW) | −0.356 | −0.298 | −0.417 | |
| SF·(BLANKTAPE − BLANKTW) | −7.930 | −8.207 | −8.725 | |
| Turbidity Offset | WET Labs, Inc. ECO-FLNTUS Sensors | |||
|---|---|---|---|---|
| s/n | 2776 | 3372 | 615 | |
| SF·( BLANKTAPE– BLANKAIR) | –2.003 | –2.169 | –2.326 | |
| SF·( BLANKTAPE– BLANKFSW) | –1.497 | –1.867 | –1.597 | |
| SF·( BLANKTAPE– BLANKFSWB) | –0.404 | –0.270 | –0.290 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pensieri, S.; Bozzano, R.; Schiano, M.E.; Ntoumas, M.; Potiris, E.; Frangoulis, C.; Podaras, D.; Petihakis, G. Methods and Best Practice to Intercompare Dissolved Oxygen Sensors and Fluorometers/Turbidimeters for Oceanographic Applications. Sensors 2016, 16, 702. https://doi.org/10.3390/s16050702
Pensieri S, Bozzano R, Schiano ME, Ntoumas M, Potiris E, Frangoulis C, Podaras D, Petihakis G. Methods and Best Practice to Intercompare Dissolved Oxygen Sensors and Fluorometers/Turbidimeters for Oceanographic Applications. Sensors. 2016; 16(5):702. https://doi.org/10.3390/s16050702
Chicago/Turabian StylePensieri, Sara, Roberto Bozzano, M. Elisabetta Schiano, Manolis Ntoumas, Emmanouil Potiris, Constantin Frangoulis, Dimitrios Podaras, and George Petihakis. 2016. "Methods and Best Practice to Intercompare Dissolved Oxygen Sensors and Fluorometers/Turbidimeters for Oceanographic Applications" Sensors 16, no. 5: 702. https://doi.org/10.3390/s16050702