Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids
Abstract
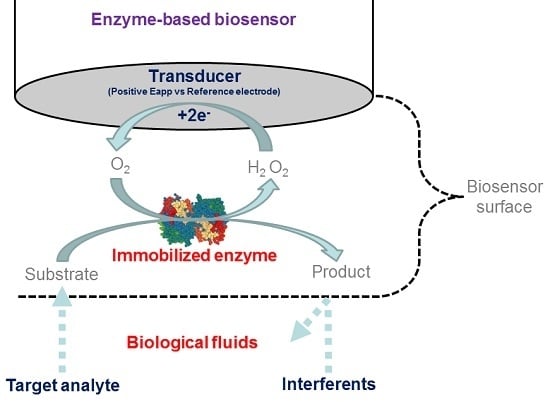
:1. Introduction
- As “off line” devices — biological samples are collected and target analytes are measured using biosensor-based analytical equipment. For example, commercial devices are available for measuring blood glucose.
- As “in vivo” sensors — biosensors are implanted and continuously detect extracellular changes in the concentrations of the analyte of interest. The invasiveness of such implantable devices limits their use mainly to preclinical research in animal models.
- As “on-line” device — biosensors are integrated with a sampling device implanted in the body or biological material. For instance, microdialysis probes can be implanted and connected to a flow through detector incorporating a biosensor element.
2. Amperometric Enzyme Biosensors
2.1. First Generation Biosensors
2.2. Second Generation Biosensors
2.3. Third Generation Biosensors
2.4. pH and Temperature Dependence
2.5. Enzyme as Label Element Instead of Recognition Element
3. Enzyme Biosensor Analytical Performance over Time
3.1. Biofouling, Electrode Passivation, Enzyme Inactivation and Loss
3.2. Strategies to Increase Enzyme Selectivity, Specificity and Lifetime
3.2.1. Adsorption
3.2.2. Sol–Gel Process
3.2.3. Covalent Binding
3.2.4. Polymeric Films
3.2.5. Enzyme Stabilizers
4. Biological Matrices: Composition and Matrix-Related Detection Problems
4.1. Saliva
4.2. Urine
4.3. Blood, Plasma and Serum
4.4. Extracellular Fluid (ECF) and Brain Extracellular Fluid (bECF)
4.5. Tears
4.6. Sweat
4.7. Changes in Biological Fluids Composition Related to Physiological and Pathological Conditions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al Hariri, M.; Lee, Y.K.; Jaffa, A.A. Recent advances in application of biosensors in tissue engineering. Biomed. Res. Int. 2014, 2014, 307519. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [Green Version]
- Lowe, R.S. Overview of Biosensor and Bioarray Technologies. In Handbook of Biosensors and Biochips; Wiley: Weinheim, Germany, 2007. [Google Scholar]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N.Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Skládal, P. Electrochemical biosensors-principles and applications. J. Appl. Biomed. 2008, 6, 57–64. [Google Scholar]
- D’Orazio, P. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69. [Google Scholar] [CrossRef]
- Wilson, M.S. Electrochemical immunosensors for the simultaneous detection of two tumor markers. Anal. Chem. 2005, 77, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Real-time electrochemical monitoring: toward green analytical chemistry. Acc. Chem. Res. 2002, 35, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Švorc, J.; Miertuš, S.; Katrlík, J.; Stred’anský, M. Composite Transducers for Amperometric Biosensors. The Glucose Sensor. Anal. Chem. 1997, 69, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [PubMed]
- Pingarròn, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- O’Neill, R.D.; Chang, S.C.; Lowry, J.P.; McNeil, C.J. Comparisons of platinum, gold, palladium and glassy carbon as electrode materials in the design of biosensors for glutamate. Biosens. Bioelectron. 2004, 19, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, P.J.; Ding, J.; Liu, J. Aptamer-based biosensors for biomedical diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Gáspár, S.; Leth, S.; Niculescu, M.; Mortari, A.; Bontidean, I.; Soukharev, V.; Dorneanu, S.A.; Ryabov, A.D.; Csöregi, E. Biosensors for life quality: Design, development and applications. Sens. Actuators B Chem. 2004, 102, 179–194. [Google Scholar] [CrossRef]
- Belluzo, M.S.; Ribone, M.E.; Lagier, C.M. Assembling Amperometric Biosensors for Clinical Diagnostics. Sensors 2008, 8, 1366–1399. [Google Scholar] [CrossRef]
- Corrie, S.R.; Coffey, J.W.; Islam, J.; Markey, K.A.; Kendall, M.A. Blood, sweat, and tears: Developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 2015, 140, 4350–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.C.; Stevens, A.L.; Han, J. Million-fold preconcentration of proteins and peptides by nanofluidic filter. Anal. Chem. 2005, 77, 4293–4299. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, S.B.; Ramasamy, R.; Gopal, N.; Kuzhandaivelu, V. Biosensors in clinical chemistry: An overview. Adv. Biomed. Res. 2014, 3, 67. [Google Scholar] [PubMed]
- Lee, Y.H.; Mutharasan, R. Biosensors. In Sensors Technology Handbook; Elsevier: Amsterdam, Netherlands, 2005; pp. 161–180. [Google Scholar]
- Dzyadevych, S.V.; Arkhypova, V.N.; Soldatkin, A.P.; El’skaya, A.V.; Martelet, C.; Jaffrezi-Renault, N. Amperometric enzyme biosensors: Past, present and future. IRBM 2008, 29, 171–180. [Google Scholar] [CrossRef]
- Harper, A.; Anderson, M.R. Electrochemical Glucose Sensors—Developments Using Electrostatic Assembly and Carbon Nanotubes for Biosensor Construction. Sensors 2010, 10, 8248–8274. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Unnikrishnan, B.; Chen, S. An Amperometric Biosensor Based on Direct Immobilization of Horseradish Peroxidase on Electrochemically Reduced Graphene Oxide Modified Screen Printed Carbon Electrode. Int. J. Electrochem. Sci. 2012, 7, 7935–7947. [Google Scholar]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.P.; Rocchitta, G.; Serra, P.A.; Kirwan, S.M.; Lowry, J.P.; O’Neill, R.D. Control of the oxygen dependence of an implantable polymer/enzyme composite biosensor for glutamate. Anal. Chem. 2006, 78, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.F.; Park, J.Y. Plain to point network reduced graphene oxide—Activated carbon composites decorated with platinum nanoparticles for urine glucose detection. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, Ö.; Kızılkaya, B.; Uysal, H.; Dilgin, Y. Biosensing of glucose in flow injection analysis system based on glucose oxidase-quantum dot modified pencil graphite electrode. Talanta 2016. [Google Scholar] [CrossRef] [PubMed]
- Devasenathipathy, R.; Mani, V.; Chen, S.M.; Huang, S.T.; Huang, T.T.; Lin, C.M.; Hwa, K.Y.; Chen, T.Y.; Chen, B.J. Glucose biosensor based on glucose oxidase immobilized at gold nanoparticles decorated graphene-carbon nanotubes. Enzyme Microb. Technol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Secchi, O.; Alvau, M.D.; Farina, D.; Bazzu, G.; Calia, G.; Migheli, R.; Desole, M.S.; O’Neill, R.D.; Serra, P.A. Simultaneous telemetric monitoring of brain glucose and lactate and motion in freely moving rats. Anal. Chem. 2013, 85, 10282–10288. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, Ş.; Aynacı, E.; Arslan, F. An amperometric biosensor for L-glutamate determination prepared from L-glutamate oxidase immobilized in polypyrrole-polyvinylsulphonate film. Artif. Cells Nanomed. Biotechnol. 2016, 44, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Soldatkin, O.; Nazarova, A.; Krisanova, N.; Borуsov, A.; Kucherenko, D.; Kucherenko, I.; Pozdnyakova, N.; Soldatkin, A.; Borisova, T. Monitoring of the velocity of high-affinity glutamate uptake by isolated brain nerve terminals using amperometric glutamate biosensor. Talanta 2015, 135, 67–74. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.P.; Rocchitta, G.; Serra, P.A.; Kirwan, S.M.; Lowry, J.P.; O’Neill, R.D. The efficiency of immobilised glutamate oxidase decreases with surface enzyme loading: An electrostatic effect, and reversal by a polycation significantly enhances biosensor sensitivity. Analyst 2006, 131, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Chinnadayyala, S.R.; Santhosh, M.; Singh, N.K.; Goswami, P. Alcohol oxidase protein mediated in-situ synthesized and stabilized gold nanoparticles for developing amperometric alcohol biosensor. Biosens. Bioelectron. 2015, 69, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Gamella, M.; Campuzano, S.; Manso, J.; González de Rivera, G.; López-Colino, F.; Reviejo, A.J.; Pingarrón, J.M. A novel non-invasive electrochemical biosensing device for in situ determination of the alcohol content in blood by monitoring ethanol in sweat. Anal. Chim. Acta 2014, 806, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Secchi, O.; Zinellu, M.; Spissu, Y.; Pirisinu, M.; Bazzu, G.; Migheli, R.; Desole, M.S.; O’Neill, R.D.; Serra, P.A.; Rocchitta, G. Further in vitro characterization of an implantable biosensor for ethanol monitoring in the brain. Sensors 2013, 13, 9522–9535. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Secchi, O.; Alvau, M.D.; Migheli, R.; Calia, G.; Bazzu, G.; Farina, D.; Desole, M.S.; O’Neill, R.D.; Serra, P.A. Development and characterization of an implantable biosensor for telemetric monitoring of ethanol in the brain of freely moving rats. Anal. Chem. 2012, 84, 7072–7079. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Gómez, P.; Gutiérrez-Capitán, M.; Capdevila, F.; Puig-Pujol, A.; Fernández-Sánchez, C.; Jiménez-Jorquera, C. Monitoring of malolactic fermentation in wine using an electrochemical bienzymatic biosensor for l-lactate with long term stability. Anal. Chim. Acta 2016, 905, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Andrus, L.P.; Unruh, R.; Wisniewski, N.A.; McShane, M.J. Characterization of Lactate Sensors Based on Lactate Oxidase and Palladium Benzoporphyrin Immobilized in Hydrogels. Biosensors 2015, 5, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xu, J.; Liu, M.; Li, D.; He, H. Amperometric vitamin C biosensor based on the immobilization of ascorbate oxidase into the biocompatible sandwich-type composite film. Appl. Biochem. Biotechnol. 2012, 167, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wen, Y.; Xu, J.; He, H.; Li, D.; Yue, R.; Liu, G. An amperometric biosensor based on ascorbate oxidase immobilized in poly(3,4-ethylenedioxythiophene)/multi-walled carbon nanotubes composite films for the determination of L-ascorbic acid. Anal. Sci. 2011, 27, 477. [Google Scholar] [CrossRef] [PubMed]
- Lata, K.; Dhull, V.; Hooda, V. Fabrication and Optimization of ChE/ChO/HRP-AuNPs/c-MWCNTs Based Silver Electrode for Determining Total Cholesterol in Serum. Biochem. Res. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Malik, J.; Prashant, A.; Jaiwal, P.K.; Pundir, C.S. Amperometric determination of serum total cholesterol with nanoparticles of cholesterol esterase and cholesterol oxidase. Anal. Biochem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Turner, A.P.; Tiwari, A. Cholesterol Oxidase Functionalised Polyaniline/Carbon Nanotube Hybrids for an Amperometric Biosensor. J. Nanosci. Nanotechnol. 2015, 15, 3373–3377. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Asiria, A.M. Selective choline biosensors based on choline oxidase co-immobilized into self-assembled monolayers on micro-chips at low potential. Anal. Methods 2015, 7, 9426–9434. [Google Scholar] [CrossRef]
- Tunç, A.T.; Aynacı Koyuncu, E.; Arslan, F. Development of an acetylcholinesterase-choline oxidase based biosensor for acetylcholine determination. Artif. Cells Nanomed. Biotechnol. 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivera, J.C.; Osma, J.F. Fabrication of an Amperometric Flow-Injection Microfluidic Biosensor Based on Laccase for In Situ Determination of Phenolic Compounds. Biomed. Res. Int. 2015, 2015, 845261. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Fooladi, E.; Malekaneh, M. A new amperometric biosensor based on Fe3O4/polyaniline/laccase/chitosan biocomposite-modified carbon paste electrode for determination of catechol in tea leaves. Appl. Biochem. Biotechnol. 2015, 175, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Casero, E.; Petit-Domínguez, M.D.; Vázquez, L.; Ramírez-Asperilla, I.; Parra-Alfambra, A.M.; Pariente, F.; Lorenzo, E. Laccase biosensors based on different enzyme immobilization strategies for phenolic compounds determination. Talanta 2013, 115, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Campanhã Vicentini, F.; Garcia, L.L.; Figueiredo-Filho, L.C.; Janegitz, B.C.; Fatibello-Filho, O. A biosensor based on gold nanoparticles, dihexadecylphosphate, and tyrosinase for the determination of catechol in natural water. Enzyme Microb. Technol. 2016, 84, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kochana, J.; Wapiennik, K.; Kozak, J.; Knihnicki, P.; Pollap, A.; Woźniakiewicz, M.; Nowak, J.; Kościelniak, P. Tyrosinase-based biosensor for determination of bisphenol A in a flow-batch system. Talanta 2015, 144, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Njagi, J.; Chernov, M.M.; Leiter, J.C.; Andreescu, S. Amperometric detection of dopamine in vivo with an enzyme based carbon fiber microbiosensor. Anal. Chem. 2010, 82, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Dzyadevych, S.V. Amperometric biosensors key work principles and features of transducers of different generations. Biopol. Cell. 2002, 18, 13–25. [Google Scholar] [CrossRef]
- Lee, C.A.; Tsai, Y.C. Preparation of multiwalled carbon nanotube-chitosan-alcohol dehydrogenase nanobiocomposite for amperometric detection of ethanol. Sens. Actuators B Chem. 2009, 138, 518–523. [Google Scholar] [CrossRef]
- Gómez-Anquela, C.; García-Mendiola, T.; Abad, J.M.; Pita, M.; Pariente, F.; Lorenzo, E. Scaffold electrodes based on thioctic acid-capped gold nanoparticles coordinated Alcohol Dehydrogenase and Azure A films for high performance biosensor. Bioelectrochemistry 2015, 106, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zaman, M.H. Amperometric measurements of ethanol on paper with a glucometer. Talanta 2015, 134, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, H.; Deng, L. A sensitive NADH and ethanol biosensor based on Graphene-Au nanorods nanocomposites. Talanta 2013, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, Y.; Xi, J.; Lin, S.; Chen, Y.; Lin, Y.; Chen, Z. A novel electrochemiluminescence ethanol biosensor based on tris(2,2′-bipyridine) ruthenium (II) and alcohol dehydrogenase immobilized in graphene/bovine serum albumin composite film. Biosens. Bioelectron. 2013, 41, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhang, S.; Lang, Q.; Song, J.; Han, L.; Liu, A. Amperometric l-glutamate biosensor based on bacterial cell-surface displayed glutamate dehydrogenase. Anal. Chim. Acta 2015, 884, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.Y.; Keskin, C.S. Quantitative determination of glycine in aqueous solution using glutamate dehydrogenase-immobilized glyoxal agarose beads. Appl. Biochem. Biotechnol. 2014, 172, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, A.; Shahrokhian, S.; zad, A.I.; Mohajerzadeh, S.; Vosoughi, M.; Darbari, S.; Sanaee, Z. Mediator-less highly sensitive voltammetric detection of glutamate using glutamate dehydrogenase/vertically aligned CNTs grown on silicon substrate. Biosens. Bioelectron. 2012, 31, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Johnston, W.A.; Stein, V.; Kalimuthu, P.; Perez-Alcala, S.; Bernhardt, P.V.; Alexandrov, K. Engineering PQQ-glucose dehydrogenase into an allosteric electrochemical Ca2+ sensor. Chem. Commun. 2016, 52, 485–458. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yu, P.; Hao, J.; Wang, Y.; Ohsaka, T.; Mao, L. Continuous and simultaneous electrochemical measurements of glucose, lactate, and ascorbate in rat brain following brain ischemia. Anal. Chem. 2014, 86, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Kim, M.Y.; Reddy, S.S.; Cho, J.; Cho, C.H.; Jung, S.; Shim, Y.B. Electron-transfer mediator for a NAD-glucose dehydrogenase-based glucose sensor. Anal. Chem. 2013, 85, 11643–11649. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Li, L.; Tang, X.; Lang, Q.; Wang, H.; Li, F.; Shi, J.; Shen, W.; Palchetti, I.; Mascini, M.; Liu, A. Microbial surface display of glucose dehydrogenase for amperometric glucose biosensor. Biosens. Bioelectron. 2013, 45, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Jenie, S.N.; Prieto-Simon, B.; Voelcker, N.H. Development of L-lactate dehydrogenase biosensor based on porous silicon resonant microcavities as fluorescence enhancers. Biosens. Bioelectron. 2015, 74, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, S.; Rotariu, L.; Benito, A.M.; Maser, W.K.; Ben Ali, M.; Bala, C. A novel amperometric biosensor based on gold nanoparticles anchored on reduced graphene oxide for sensitive detection of L-lactate tumor biomarker. Biosens. Bioelectron. 2015, 69, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Prodromidis, M.I.; Karayannis, M.I. Enzyme Based Amperometric Biosensors for Food Analysis. Electroanalysis 2002, 14, 241–261. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Bradford, V.Q.; Whitaker, R.G. Enzyme electrode studies of glucose oxidase modified with a redox mediator. Talanta 1991, 38, 57–63. [Google Scholar] [CrossRef]
- Chaubey, A.; Malhotra, B.D. Mediated biosensors. Biosens. Bioelectron. 2002, 17, 441–456. [Google Scholar] [CrossRef]
- Xu, F. Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J. Biol. Chem. 1997, 272, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, V.; Lele, S.S. Laccase: Proprieties and applications. Bioresources 2009, 4, 1694–1717. [Google Scholar]
- Rangelova, V.; Tsankova, D.; Dimcheva, N. Soft Computing Techniques in Modelling the Influence of pH and Temperature on Dopamine Biosensor. In Intelligent and Biosensors; INTECH: Rijeka, Croatia, 2010; pp. 100–122. [Google Scholar]
- Purvis, D.; Leonardova, O.; Farmakovsky, D.; Cherkasov, V. An ultrasensitive and stable potentiometric immunosensor. Biosens. Bioelectron. 2003, 18, 1385–1390. [Google Scholar] [CrossRef]
- Li, J.; Xiao, L.T.; Zeng, G.M.; Huang, G.H.; Shen, G.L.; Yu, R.Q. Amperometric immunosensor based on polypyrrole/poly(m-pheylenediamine) multilayer on glassy carbon electrode for cytokinin N6-(Delta2-isopentenyl) adenosine assay. Anal. Biochem. 2003, 321, 89–95. [Google Scholar] [CrossRef]
- Ikebukuro, K.; Kiyohara, C.; Sode, K. Electrochemical Detection of Protein Using a Double Aptamer Sandwich. Anal. Lett. 2004, 37, 2901–2909. [Google Scholar] [CrossRef]
- Centi, S.; Tombelli, S.; Minunni, M.; Mascini, M. Aptamer-based detection of plasma proteins by an electrochemical assay coupled to magnetic beads. Anal. Chem. 2007, 79, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, N.; Reichert, M. Methods for reducing biosensor membrane biofouling. Colloids Surf. B Biointerfaces 2000, 18, 197–219. [Google Scholar] [CrossRef]
- Ferreira, M.; Varela, H.; Torresi, R.M.; Tremiliosi-Filho, G. Electrode passivation caused by polymerization of different phenolic compounds. Electrochim. Acta 2006, 52, 434–442. [Google Scholar] [CrossRef]
- Yang, X.; Kirsch, J.; Fergus, J.; Simonian, A. Modeling analysis of electrode fouling during electrolysis of phenolic compounds. Electrochim. Acta 2013, 94, 259–268. [Google Scholar] [CrossRef]
- Yamauchi, S. Chemical Sensor Technology. In Handbook of Biosensors and Electronic Noses: Medicine, Food, and the Environment; AEG: Frankfurt, Germany, 1997. [Google Scholar]
- O’Hare, D. Biosensors and Sensor Systems. In Body Sensor Networks; Springer: New York, NY, USA, 2014. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Cardosi, M.; Liu, Z. Amperometric Glucose Sensors for Whole Blood Measurement Based on Dehydrogenase Enzymes. In Dehydrogenases; Canuto, R.A., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Curulli, A.; Palleschi, G. Construction and application of highly selectively sensors and biosensors using non-conducting electropolymerized films. In Proceedings of the 2nd Workshop on Chemical Sensors and Biosensors, Rome, Italy, 18–19 March 2000.
- Gouda, M.D.; Kumar, M.A.; Thakur, M.S.; Karantha, N.G. Enhancement of operational stability of an enzyme biosensor for glucose and sucrose using protein based stabilizing agent. Biosens. Bioelectron. 2002, 17, 503–507. [Google Scholar] [CrossRef]
- Gibson, T.D. Biosensors: The stabilité problem. Analusis 1999, 27, 630–638. [Google Scholar] [CrossRef]
- Chaniotakis, N.A. Enzyme stabilization strategies based on electrolytes and polyelectrolytes for biosensor applications. Anal. Bioanal. Chem. 2004, 378, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a Strategy for Improving Enzyme Properties-Application to Oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, D.C.; Yeon, K.M.; Kim, S.R.; Lee, C.H. Enzyme-immobilized nanofiltration membrane to mitigate biofouling based on quorum quenching. Environ. Sci. Technol. 2011, 45, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Datta, M.; Malhotra, B.D. Recent advances in cholesterol biosensor. Biosens. Bioelectron. 2008, 23, 1083–1100. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, S.; Marty, J.L. Twenty years research in cholinesterase biosensors: from basic research to practical applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.F.M. Progress in enzyme-based biosensors using optical transducers. Microchim. Acta 2004, 148, 107–132. [Google Scholar] [CrossRef]
- Ambrozy, A.; Hlavata, L.; Labuda, J. Protective membranes at electrochemical biosensors. Acta Chim. Slovaca 2013, 6, 35–41. [Google Scholar] [CrossRef]
- Dixon, B.M.; Lowry, J.P.; O’Neill, R.D. Characterization in vitro and in vivo of the oxygen dependence of an enzyme/polymer biosensor for monitoring brain glucose. J. Neurosci. Methods 2002, 119, 135–142. [Google Scholar] [CrossRef]
- Rothwell, S.A.; Killoran, S.J.; O’Neill, R.D. Enzyme immobilization strategies and electropolymerization conditions to control sensitivity and selectivity parameters of a polymer-enzyme composite glucose biosensor. Sensors 2010, 10, 6439–6462. [Google Scholar] [CrossRef] [PubMed]
- Livage, J.; Coradin, T.; Roux, C. Encapsulation of biomolecules in silica gels. J. Phys. Condens. Matter. 2001, 13, 673–691. [Google Scholar] [CrossRef]
- Reetz, M.T.; Wenkel, R.; Avnir, D. Entrapment of lipases in hydrophobic sol-gel materials: Efficient heterogenous biocatalysts in aqueous medium. Synthesis 2000, 6, 781–783. [Google Scholar] [CrossRef]
- Gill, I.; Ballesteros, A. Bioencapsulation within synthetic polymers (Part 1): Sol-gel encapsulated biologicals. Trends Biotechnol. 2000, 18, 282–296. [Google Scholar] [CrossRef]
- Avnir, D.; Braun, S.; Lev, O.; Ottolenghi, M. Enzymes and other proteins entrapped in sol-gel materials. Chem. Mater. 1994, 6, 1605–1614. [Google Scholar] [CrossRef]
- Briones, M.; Casero, E.; Vázquez, L.; Pariente, F.; Lorenzo, E.; Petit-Domínguez, M.D. Diamond nanoparticles as a way to improve electron transfer in sol-gel L-lactate biosensing platforms. Anal. Chim. Acta 2016, 908, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Eggins, B.R. Chemical Sensors and Biosensors; John Wiley & Sons: West Sussex, UK, 2002. [Google Scholar]
- Abian, O.; Wilson, L.; Mateo, C.; Fernández-Lorente, G.; Palomo, J.M.; Fernández-Lafuente, R.; Guisán, J.M.; Re, D.; Tam, A.; Daminatti, M. Preparation of artificial hyper-hydrophilic micro-environments (polymeric salts) surrounding enzyme molecules: New enzyme derivatives to be used in any reaction medium. J. Mol. Catal. B Enzym. 2002, 19–20, 295–303. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S. Polymers in Biosensors. In Biomedical Polymers; Woodhead Publishing: Cambridge, UK, 2007. [Google Scholar]
- Yuan, C.J.; Hsu, C.L.; Wang, S.C.; Changb, K.S. Eliminating the Interference of Ascorbic Acid and Uric Acid to the Amperometric Glucose Biosensor by Cation Exchangers Membrane and Size Exclusion Membrane. Electroanalysis 2005, 17, 2239–2245. [Google Scholar] [CrossRef]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Development of an amperometric screen-printed galactose biosensor for serum analysis. Anal. Biochem. 2013, 435, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.; Consales, M.; Severino, R.; Vaiano, P.; Boniello, A.; Sandomenico, A.; Ruvo, M.; Borriello, A.; Diodato, L.; Zuppolini, S.; et al. Long period fiber grating nano-optrode for cancer biomarker detection. Biosens. Bioelectron. 2016, 80, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Gutknecht, J. Permeability of small nonelectrolytes through lipid bilayer membranes. J. Membr. Biol. 1986, 90, 207–217. [Google Scholar] [CrossRef]
- Rothwell, S.A.; Kinsella, M.E.; Zain, Z.M.; Serra, P.A.; Rocchitta, G.; Lowry, J.P.; O’Neill, R.D. Contributions by a novel edge effect to the permselectivity of an electrosynthesized polymer for microbiosensor applications. Anal. Chem. 2009, 81, 3911–3918. [Google Scholar] [CrossRef] [PubMed]
- Vaddiraju, S.; Burgess, D.J.; Jain, F.C.; Papadimitrakopoulos, F. The role of H2O2 outer diffusion on the performance of implantable glucose sensors. Biosens. Bioelectron. 2009, 24, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.A.; Vaddiraju, S.; Papadimitrakopoulos, F.; Jain, F.C. Theoretical Analysis of the Performance of Glucose Sensors with Layer-by-Layer Assembled Outer Membranes. Sensors 2012, 12, 13402–13416. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, S.A.; O’Neill, R.D. Effects of applied potential on the mass of non-conducting poly(ortho-phenylenediamine) electro-deposited on EQCM electrodes: comparison with biosensor selectivity parameters. Phys. Chem. Chem. Phys. 2011, 13, 5413–5421. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, S.M.; Rocchitta, G.; McMahon, C.P.; Craig, J.D.; Killoran, S.J.; O’Brien, K.B.; Serra, P.A.; Lowry, J.P.; O’Neill, R.D. Modifications of Poly(o-phenylenediamine) Permselective Layer on Pt-Ir for Biosensor Application in Neurochemical Monitoring. Sensors 2007, 7, 420–437. [Google Scholar] [CrossRef]
- McMahon, C.P.; Killoran, S.J.; Kirwan, S.M.; O’Neill, R.D. The selectivity of electrosynthesised polymer membranes depends on the electrode dimensions: Implications for biosensor applications. Chem. Commun. 2004, 18, 2128–2130. [Google Scholar] [CrossRef] [PubMed]
- Calia, G.; Monti, P.; Marceddu, S.; Dettori, M.A.; Fabbri, D.; Jaoua, S.; O’Neill, R.D.; Serra, P.A.; Delogu, G.; Migheli, Q. Electropolymerized phenol derivatives as permselective polymers for biosensor applications. Analyst 2015, 140, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, C.; Gao, R.; Xia, C.; Yuan, G.; Li, Y.; Zhao, Y.; Chen, Q.; He, J. A novel DNA biosensor integrated with Polypyrrole/streptavidin and Au-PAMAM-CP bionanocomposite probes to detect the rs4839469 locus of the vangl1 gene for dysontogenesis prediction. Biosens. Bioelectron. 2016, 80, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Ayenimo, J.G.; Adeloju, S.B. Rapid amperometric detection of trace metals by inhibition of an ultrathin polypyrrole-based glucose biosensor. Talanta 2016, 148, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Palod, P.A.; Singh, V. Improvement in glucose biosensing response of electrochemically grown polypyrrole nanotubes by incorporating crosslinked glucose oxidase. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, J.; Huo, X.; Yan, R.; Wong, D.K. An intimately bonded titanate nanotube-polyaniline-gold nanoparticle ternary composite as a scaffold for electrochemical enzyme biosensors. Anal. Chim. Acta 2016, 911, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Yoon, H.H. Amperometric urea biosensors based on sulfonated graphene/polyaniline nanocomposite. Int. J. Nanomed. 2015, 10, 55–66. [Google Scholar]
- Cui, M.; Song, Z.; Wu, Y.; Guo, B.; Fan, X.; Luo, X. A highly sensitive biosensor for tumor maker alpha fetoprotein based on poly(ethylene glycol) doped conducting polymer PEDOT. Biosens. Bioelectron. 2016, 79, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Galán, T.; Prieto-Simón, B.; Alvira, M.; Eritja, R.; Götz, G.; Bäuerle, P.; Samitier, J. Label-free electrochemical DNA sensor using “click”-functionalized PEDOT electrodes. Biosens. Bioelectron. 2015, 74, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Wang, X.; Uchiyama, S. Polymers for Biosensors Construction. In State of the Art in Biosensors—General Aspects; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Yano, K.; Karube, I. Molecularly imprinted polymers for biosensor applications. TrAC Trends Anal. Chem. 1999, 18, 199–204. [Google Scholar] [CrossRef]
- Gavalas, V.G.; Chaniotakis, N.A.; Gibson, T.D. Improved operational stability of biosensors based on enzyme-polyelectrolyte complex adsorbed into a porous carbon electrode. Biosens. Bioelectron. 1998, 13, 1205–1211. [Google Scholar] [CrossRef]
- Gibson, T.D.; Higgins, I.J.; Woodward, J.R. Stabilization of analytical enzymes using a novel polymer-carbonate system and the production of a stabilized, single reagent for alcohol analysis. Analyst 1992, 117, 1293–1297. [Google Scholar] [CrossRef]
- Rochefort, D.; Kouisni, L.; Gendron, K. Physical immobilization of laccase on an electrode by means of poly(ethyleneimine) microcapsules. J. Electroanal. Chem. 2008, 217, 53–63. [Google Scholar] [CrossRef]
- Belay, A.; Collins, A.; Ruzgas, T.; Kissinger, P.T.; Gorton, L.; Csöregi, E. Redox hydrogel based bienzyme electrode for L-glutamate monitoring. J. Pharm Biomed. Anal. 1999, 19, 93–105. [Google Scholar] [CrossRef]
- McMahon, C.P.; Rocchitta, G.; Kirwan, S.M.; Killoran, S.J.; Serra, P.A.; Lowry, J.P.; O’Neill, R.D. Oxygen tolerance of an implantable polymer/enzyme composite glutamate biosensor displaying polycation-enhanced substrate sensitivity. Biosens. Bioelectron. 2007, 22, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.A.; Hensley, P.M.; Loch, C.L. Evaluation of Polycation-Stabilized Lactate Oxidase in a Silica Sol–Gel as a Biosensor Platform. Microchim. Acta 2003, 142, 1–5. [Google Scholar] [CrossRef]
- Bradbury, S.L.; Jakoby, W.B. Glycerol as an Enzyme-Stabilizing Agent: Effects on Aldehyde Dehydrogenase. Proc. Nat. Acad. Sci. USA 1972, 69, 2373–2376. [Google Scholar] [CrossRef] [PubMed]
- Adkins, J.N.; Varnum, S.M.; Auberry, K.J.; Moore, R.J.; Angell, N.H.; Smith, R.D.; Springer, D.L.; Pounds, J.G. Toward a human blood serum proteome: Analysis by multidimensional separation coupled with mass spectrometry. Mol. Cell. Proteom. 2002, 1, 947–955. [Google Scholar] [CrossRef]
- Papadimitrakopoulos, F.; Vaddiraju, S. Control of Biofouling in Implantable Biosensors. U.S. Patent 9101301 B2, 15 August 2015. [Google Scholar]
- Padiglia, A.; Medda, R.; Lorrai, A.; Paci, M.; Pedersen, J.Z.; Boffi, A.; Bellelli, A.; Finazzi Agro, A.; Floris, G. Irreversible inhibition of pig kidney copper-containing amine oxidase by sodium and lithium ions. J. Eur. Biochem. 2001, 268, 4686–4697. [Google Scholar] [CrossRef]
- Atta, A.; Akhtar, M.S.; Bhakuni, V. Monovalent Cation-Induced Conformational Change in Glucose Oxidase Leading to Stabilization of the Enzyme. Biochemistry 2001, 40, 1945–1955. [Google Scholar]
- Borgmann, S.; Schulte, A.; Neugebauer, S.; Schuhmann, W. Amperometric Biosensors. In Advances in Electrochemical Science and Engineering; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Sasso, S.V.; Pierce, R.J.; Walla, R.; Yacynych, A.M. Electropolymerized 1,2-diaminobenzene as a means to prevent interferences and fouling and to stabilize immobilized enzyme in electrochemical biosensors. Anal. Chem. 1990, 62, 1111–1117. [Google Scholar] [CrossRef]
- Geise, R.J.; Adams, J.M.; Barone, N.J.; Yacynych, A.M. Electropolymerized films to prevent interferences and electrode fouling in biosensors. Biosens. Bioelectron. 1991, 6, 151–160. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T.J. A review of saliva: Normal composition, flow, and function. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Mitsumori, M.; Kano, Y. Noninvasively measuring blood glucose using saliva: A bloodless procedure based on an enzyme-sensor system. IEEE Eng. Med. Biol. 1998, 17, 59–63. [Google Scholar] [CrossRef]
- Guilbault, G.G.; Palleschi, G.; Lubrano, G. Non-invasive biosensors in clinical analysis. Biosens. Bioelectron. 1995, 10, 379–392. [Google Scholar] [CrossRef]
- Butterworth, P.J.; Warren, F.J.; Ellis, P.R. Human a-amylase and starch digestion: An interesting marriage. Starch Starke 2011, 63, 395–405. [Google Scholar] [CrossRef]
- Baig, A. Biochemical Composition of Normal Urine. Nat. Precedings 2011. [Google Scholar] [CrossRef]
- Blood, Plasma, and Cellular Blood Components. Available online: http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/chapter5.pdf (accessed on 29 February 2016).
- Di Chiara, G. Brain Dialysis of Monoamines. In Microdialysis in the Neurosciences; Robinson, T.E., Justice, J.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- O’Neill, R.D. Microvoltammetric techniques and sensors for monitoring neurochemical dynamics in vivo. A review. Analyst 1994, 119, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Calia, G.; Rocchitta, G.; Migheli, R.; Puggioni, G.; Spissu, Y.; Bazzu, G.; Mazzarello, V.; Lowry, J.P.; O’Neill, R.D.; Desole, M.S.; et al. Biotelemetric monitoring of brain neurochemistry in conscious rats using microsensors and biosensors. Sensors 2009, 9, 2511–2523. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Alvau, M.D.; Puggioni, G.; Calia, G.; Bazzu, G.; Migheli, R.; Secchi, O.; Rocchitta, G.; Desole, M.S.; Serra, P.A. Implantable (Bio)sensors as new tools for wireless monitoring of brain neurochemistry in real time. World J. Pharmacol. 2014, 3, 1–17. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Robinson, A.H. Chemical Composition of Sweat. Physiol. Rev. 1954, 34, 202–220. [Google Scholar] [PubMed]
- Ikawa, M.; Okazawa, H.; Kudo, T.; Kuriyama, M.; Fujibayashi, Y.; Yoneda, M. Evaluation of striatal oxidative stress in patients with Parkinson’s disease using [62Cu] ATSM PET. Nuclear Med. Biol. 2011, 38, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Reinke, S.N.; Mamik, M.K.; McKenzie, B.A.; Maingat, F.; Branton, W.G.; Broadhurst, D.I.; Christopher, P. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology 2014, 11, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, P.; Rugarli, E.I. Emerging roles of mitochondrial proteases in neurodegeneration. Biochim. Biophys. Acta 2010, 1197, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pejler, G.; Ronnberg, E.; Waern, I.; Wernersson, S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood 2010, 115, 4981–4990. [Google Scholar] [CrossRef] [PubMed]
- DeClerck, Y.A.; Mercurio, A.M.; Stack, M.S.; Chapman, H.A.; Zutter, M.M.; Muschel, R.J.; Raz, A.; Matrisian, L.M.; Sloane, B.F.; Noel, A.; et al. Proteases, Extracellular Matrix, and Cancer. Am. J. Pathol. 2004, 164, 1131–1139. [Google Scholar] [CrossRef]
- Felix, K.; Gaida, M.M. Neutrophil-Derived Proteases in the Microenvironment of Pancreatic Cancer—Active Players in Tumor Progression. Int. J. Biol. Sci. 2016, 12, 302–313. [Google Scholar] [CrossRef] [PubMed]





| Enzyme | Source | Substrate | References |
|---|---|---|---|
| Glucose oxidase | Aspergillus niger, E.C. 1.1.3.4 | β-d-Glucose | [25,26,27,28] |
| Glutamate oxidase | Streptomyces sp EC 1.4.3.11 | l-Glutamate | [24,29,30,31] |
| Alcohol oxidase | Pichia pastoris Hansenula polymorpha EC 1.1.3.13 | Ethanol | [32,33,34,35] |
| Lactate oxidase | Pediococcus sp. Aerococcus viridians EC 1.1.3.2 | l-Lactate | [28,36,37,38] |
| Ascorbate oxidase | Cucurbita sp EC 1.10.3.3 | l-Ascorbic acid | [39,40] |
| Cholesterol oxidase | Streptomyces sp porcine pancreas EC 1.1.3.6 | Cholesterol | [41,42,43] |
| Choline Oxidase | Alcaligenes sp (EC 1.1.3.17) | Choline Acetylcholine | [44,45] |
| Laccase | Trametes pubescens Paraconiothyrium variable Trametes versicolor (EC 1.1.3.4) | Polyphenols | [46,47,48] |
| Tyrosinase | Mushroom EC 1.14.18.1 | Monophenols Dihydroxyphenols Bisphenol A | [49,50,51] |
| Enzyme | Source | Substrate | References |
|---|---|---|---|
| Alcohol dehydrogenase | Saccharomyces cerevisiae E.C. 1.1.1.1 | Etanol | [54,55,56,57] |
| Glutamate dehydrogenase | bovine liver E.C. 1.4.1.2 | l-Glutamate | [58,59,60] |
| Glucose dehydrogenase | Pseudomonas sp. Escherichia coli EC 1.1.1.47 | Glucose | [61,62,63,64] |
| Lactate dehydrogenase | Rabbit muscle Chicken heart EC 1.1.1.27 | l-Lactate | [62,65,66] |
| Fluid | Cations | Anions | Proteins | Metabolites | Nutrients |
|---|---|---|---|---|---|
| Saliva | ++ | +++ | ++ | −−− | −−− |
| Urine | ++ | +++ | −− | +++ | −−−− |
| Blood | ++ | ++ | +++ | +++ | +++ |
| ECF | ++++ | +++++ | −− | ++ | + |
| Tears | ++ | ++ | −− | + | + |
| Sweat | +++ | +++ | −− | + | + |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. https://doi.org/10.3390/s16060780
Rocchitta G, Spanu A, Babudieri S, Latte G, Madeddu G, Galleri G, Nuvoli S, Bagella P, Demartis MI, Fiore V, et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors. 2016; 16(6):780. https://doi.org/10.3390/s16060780
Chicago/Turabian StyleRocchitta, Gaia, Angela Spanu, Sergio Babudieri, Gavinella Latte, Giordano Madeddu, Grazia Galleri, Susanna Nuvoli, Paola Bagella, Maria Ilaria Demartis, Vito Fiore, and et al. 2016. "Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids" Sensors 16, no. 6: 780. https://doi.org/10.3390/s16060780