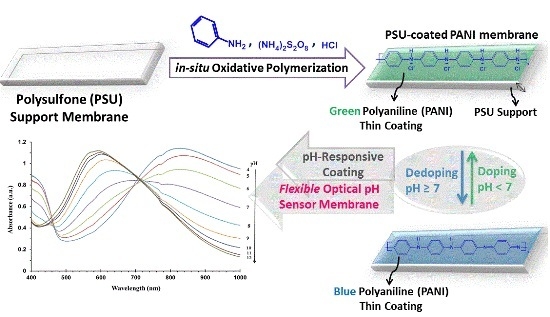
A Flexible Optical pH Sensor Based on Polysulfone Membranes Coated with pH-Responsive Polyaniline Nanofibers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PANI-Coated PSU Sensor Membranes
2.3. Sensor Characterization and pH Measurements
2.4. Instrumentation
3. Results and Discussion
3.1. Optimization of the Reaction Conditions
3.2. Thickness of Polyaniline Coatings
3.3. FTIR Analysis
3.4. SEM Analysis
3.5. Performance of PANI-Coated PSU Membranes as Optical pH Chemical Sensor
3.6. pH and pKa Measurements
3.7. Hysteresis
3.8. Response Time
3.9. Storage, Stability and Reproducibility of PANI-Coated PSU Sesnor Membrane
3.10. Real Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef] [PubMed]
- Qazi, H.H.; Mohammad, A.B.B.; Akram, M. Recent progress in optical chemical sensors. Sensors 2012, 12, 16522–16556. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kim, Y.-G.; Drew, C.; Ku, B.-C.; Kumar, J.; Samuelson, L.A. Electrostatic assembly of conjugated polymer thin layers on electrospun nanofibrous membranes for biosensors. Nano Lett. 2004, 4, 331–334. [Google Scholar] [CrossRef]
- Borisov, S.M.; Wolfbeis, O.S. Optical biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Chen, L.; Qin, W. Potentiometric detection of chemical vapors using molecularly imprinted polymers as receptors. Sci. Rep. 2015, 5, 12462. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.-J.; Zou, X.U.; Hamedi, M.M.; Hu, J.; Parolo, C.; Maxwell, E.J.; Bühlmann, P.; Whitesides, G.M. Paper-Based Potentiometric Ion Sensing. Anal. Chem. 2014, 86, 9548–9553. [Google Scholar] [CrossRef] [PubMed]
- Malitesta, C.; Palmisano, F.; Torsi, L.; Zambonin, P.G. Glucose fast-response amperometric sensor based on glucose oxidase immobilized in an electropolymerized poly (o-phenylenediamine) film. Anal. Chem. 1990, 62, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Kros, A.; Nolte, R.J.; Sommerdijk, N. Conducting polymers with confined dimensions: Track-etch membranes for amperometric biosensor applications. Adv. Mater. 2002, 14, 1779–1782. [Google Scholar] [CrossRef]
- Heng, L.Y.; Fang, T.H.; Chern, L.H.; Ahmad, M. Influence of methacrylic-acrylic copolymer composition on plasticiser-free optode films for pH sensors. Sensors 2003, 3, 83–90. [Google Scholar] [CrossRef]
- Korostynska, O.; Arshak, K.; Gill, E.; Arshak, A. Review on State-of-the-art in Polymer Based pH Sensors. Sensors 2007, 7, 3027–3042. [Google Scholar] [CrossRef]
- Dizman, C.; Tasdelen, M.A.; Yagci, Y. Recent advances in the preparation of functionalized polysulfones. Polym. Int. 2013, 62, 991–1007. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Miyagishi, T.; Ito, A.; Matsuguchi, M.; Sadaoka, Y.; Sakai, Y. A thin-film polysulfone-based capacitive-type relative-humidity sensor. Sens. Actuators B Chem. 1995, 25, 692–695. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, S. An investigation of sulfonated polysulfone humidity-sensitive materials. Sens. Actuators A Phys. 1994, 40, 147–149. [Google Scholar] [CrossRef]
- Badocco, D.; Mondin, A.; Pastore, P.; Voltolina, S.; Gross, S. Dependence of calibration sensitivity of a polysulfone/Ru(II)-Tris(4,7-diphenyl-1,10-phenanthroline)-based oxygen optical sensor on its structural parameters. Anal. Chim. Acta 2008, 627, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Florescu, M.; Katerkamp, A. Optimisation of a polymer membrane used in optical oxygen sensing. Sens. Actuators B Chem. 2004, 97, 39–44. [Google Scholar] [CrossRef]
- Sanchez, S.; Pumera, M.; Cabruja, E.; Fàbregas, E. Carbon nanotube/polysulfone composite screen-printed electrochemical enzyme biosensors. Analyst 2007, 132, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Quijano, J.; Avilés, F.; Aguilar, J.O.; Tapia, A. Strain sensing capabilities of a piezoresistive MWCNT-polysulfone film. Sens. Actuators A Phys. 2010, 159, 135–140. [Google Scholar] [CrossRef]
- Wolfbeis, O. Optical Technology until the Year 2000: An Historical Overview. In Optical Sensors; Springer: Berlin, Germany; Heidelberg, Germany, 2004; pp. 1–34. [Google Scholar]
- Clark, H.A.; Kopelman, R.; Tjalkens, R.; Philbert, M.A. Optical nanosensors for chemical analysis inside single living cells. 2. Sensors for pH and calcium and the intracellular application of PEBBLE sensors. Anal. Chem. 1999, 71, 4837–4843. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Hamilton, C.J.; Murphy, S.M.; Tighe, B.J. Polymer membranes in clinical sensor applications: I. An overview of membrane function. Biomaterials 1992, 13, 971–978. [Google Scholar] [CrossRef]
- Lin, J. Recent development and applications of optical and fiber-optic pH sensors. TrAC Trends Anal. Chem. 2000, 19, 541–552. [Google Scholar] [CrossRef]
- Wencel, D.; Abel, T.; McDonagh, C. Optical chemical pH sensors. Anal. Chem. 2013, 86, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Worlinsky, J.L.; Halepas, S.; Ghandehari, M.; Khalil, G.; Brückner, C. High pH sensing with water-soluble porpholactone derivatives and their incorporation into a Nafion® optode membrane. Analyst 2015, 140, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Timbó, Á.P.; Pinto, P.V.F.; Pinho, H.A.; de Moura, L.P.; Chretien, J.B.; Viana, F.W.; Filho, R.G.D.; da Silva, E.B.; da Silva, M.E.R.; Menezes, J.W.M.; et al. PH optical sensor based on thin films of sol–gel with bromocresol purple. Sens. Actuators B Chem. 2016, 223, 406–410. [Google Scholar] [CrossRef]
- Ferrari, L.; Rovati, L.; Fabbri, P.; Pilati, F. Disposable fluorescence optical pH sensor for near neutral solutions. Sensors 2012, 13, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Liu, D.; Liu, X.; Guan, S.; Shi, F.; Chang, H.; He, H.; Yang, G. Fluorescent pH Sensors for Broad-Range pH Measurement Based on a Single Fluorophore. Anal. Chem. 2015, 87, 5897–5904. [Google Scholar] [CrossRef] [PubMed]
- Purdey, M.; Thompson, J.; Monro, T.; Abell, A.; Schartner, E. A Dual Sensor for pH and Hydrogen Peroxide Using Polymer-Coated Optical Fibre Tips. Sensors 2015, 15, 31904–31913. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Shepherd, R.; Diamond, D.; Diamond, D. Solid State pH Sensor Based on Light Emitting Diodes (LED) As Detector Platform. Sensors 2006, 6, 848–859. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Ahmad, M.; Taib, M.N. Applications of artificial neural network on signal processing of optical fibre pH sensor based on bromophenol blue doped with sol–gel film. Sens. Actuators B Chem. 2003, 90, 182–188. [Google Scholar] [CrossRef]
- Chen, X.; Gu, Z. Absorption-type optical pH sensitive film based on immobilized purple cabbage pigment. Sens. Actuators B Chem. 2013, 178, 207–211. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.-W. Self-calibration of a polyaniline nanowire-based chemiresistive pH sensor. Microelectron. Eng. 2014, 116, 26–32. [Google Scholar] [CrossRef]
- Chiam, Y.S.; Ahad, I.Z.M.; Harun, S.W.; Gan, S.N.; Phang, S.W. Effects of the Dopant Ratio on Polyaniline Coated Fiber Bragg Grating for pH detection. Synth. Metals 2016, 211, 132–141. [Google Scholar] [CrossRef]
- Huang, G.-W.; Xiao, H.-M.; Fu, S.-Y. Electrical switch for smart pH self-adjusting system based on silver nanowire/polyaniline nanocomposite film. ACS Nano 2015, 9, 3234–3242. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, E.; Zimin, D.; Wolfbeis, O.S. Fluorescent beads coated with polyaniline: A novel nanomaterial for optical sensing of pH. Adv. Mater. 2001, 13, 819–822. [Google Scholar] [CrossRef]
- Lindfors, T.; Harju, L.; Ivaska, A. Optical pH measurements with water dispersion of polyaniline nanoparticles and their redox sensitivity. Anal. Chem. 2006, 78, 3019–3026. [Google Scholar] [CrossRef] [PubMed]
- Mihai, I.; Addiego, F.; Ruch, D.; Ball, V. Composite and free standing PANI-PVA membranes as flexible and stable optical pH sensors. Sens. Actuators B Chem. 2014, 192, 769–775. [Google Scholar] [CrossRef]
- Tanwar, S.; Ho, J.-A.A. Green Synthesis of Novel Polyaniline Nanofibers: Application in pH Sensing. Molecules 2015, 20, 18585–18596. [Google Scholar] [CrossRef] [PubMed]
- Florea, L.; Fay, C.; Lahiff, E.; Phelan, T.; O’Connor, N.E.; Corcoran, B.; Diamond, D.; Benito-Lopez, F. Dynamic pH mapping in microfluidic devices by integrating adaptive coatings based on polyaniline with colorimetric imaging techniques. Lab Chip 2013, 13, 1079–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorova, S.; Stejskal, J. Surface and precipitation polymerization of aniline. Langmuir 2002, 18, 5630–5632. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Makhlouf, A.S.H. Smart textiles supercapacitors coated with conducting polymers for energy storage applications. In Industrial Applications for Intelligent Polymers and Coatings; Makhlouf, A.S.H., Ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Trey, S.; Jafarzadeh, S.; Johansson, M. In situ polymerization of polyaniline in wood veneers. ACS Appl. Mater. Interfaces 2012, 4, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Sapurina, I.Y.; Stejskal, J. The effect of pH on the oxidative polymerization of aniline and the morphology and properties of products. Russ. Chem. Rev. 2010, 79, 1123. [Google Scholar] [CrossRef]
- Sapurina, I.; Riede, A.; Stejskal, J. In-situ polymerized polyaniline films: 5. Brush-like chain ordering. Synth. Metals 2002, 129, 29–37. [Google Scholar] [CrossRef]
- Zhang, X.; Goux, W.J.; Manohar, S.K. Synthesis of polyaniline nanofibers by “nanofiber seeding”. J. Am. Chem. Soc. 2004, 126, 4502–4503. [Google Scholar] [CrossRef] [PubMed]
- Sapurina, I.; Riede, A.; Stejskal, J. In-situ polymerized polyaniline films: 3. Film formation. Synth. Metals 2001, 123, 503–507. [Google Scholar] [CrossRef]
- Ayad, M.; Shenashin, M. Film thickness studies for the chemically synthesized conducting polyaniline. Eur. Polym. J. 2003, 39, 1319–1324. [Google Scholar] [CrossRef]
- Grummt, U.-W.; Pron, A.; Zagorska, M.; Lefrant, S. Polyaniline based optical pH sensor. Anal. Chim. Acta 1997, 357, 253–259. [Google Scholar] [CrossRef]
- Chiou, N.R.; Epstein, A.J. Polyaniline nanofibers prepared by dilute polymerization. Adv. Mater. 2005, 17, 1679–1683. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.Z.; Han, B.H.; Wei, Z. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Sapurina, I.; Prokeš, J.; Zemek, J. In-situ polymerized polyaniline films. Synth. Metals 1999, 105, 195–202. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I. Polyaniline: Thin films and colloidal dispersions (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 815–826. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.; Duan, Y. An improved optical pH sensor based on polyaniline. Sens. Actuators B Chem. 2000, 71, 118–122. [Google Scholar] [CrossRef]
- Weiller, B.H.; Virji, S.; Baker, C.; Huang, J.; Li, D.; Kaner, R.B. Polyaniline Nanofibers and Composite Materials for Chemical Detection. Available online: http://www.nsti.org/publications/Nanotech/2005/pdf/324.pdf (accessed on 15 June 2016).
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Huang, J.; Kaner, R.B. The intrinsic nanofibrillar morphology of polyaniline. Chem. Commun. 2006, 4, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, E.; Terpetschnig, E.; Wolfbeis, O.S. Optical sensing of pH using thin films of substituted polyanilines. Anal. Chim. Acta 1997, 357, 247–252. [Google Scholar] [CrossRef]
- Ferrer-Anglada, N.; Kaempgen, M.; Roth, S. Transparent and flexible carbon nanotube/polypyrrole and carbon nanotube/polyaniline pH sensors. Phys. Status Solidi 2006, 243, 3519–3523. [Google Scholar] [CrossRef]
- Abu-Thabit, N.; Umar, Y.; Ratemi, E.; Ahmad, A. Polyaniline-Coated Polysulfone Membranes as Flexible Optical pH Sensors. In Proceedings of the 2nd International Electronic Conference on Sensors and Applications, Basel, Switzerland, 15–30 November 2015.
- Sapurina, I.; Tenkovtsev, A.V.; Stejskal, J. Conjugated polyaniline as a result of the benzidine rearrangement. Polym. Int. 2015, 64, 453–465. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2008, 42, 135–145. [Google Scholar] [CrossRef] [PubMed]








| Membrane Code | Reaction Time (min) | Absorbance at pH 4 (λ = 825 nm) | Estimated Coating Thickness 1 |
|---|---|---|---|
| (nm) | |||
| PANI-PSU-10 | 10 | 0.63 | 102 ± 7 |
| PANI-PSU-20 | 20 | 1.08 | 160 ± 9 |
| Sample | Color | pH Sensor | pH Meter | % Error 1 |
|---|---|---|---|---|
| 0.10 M NaHCO3 | Colorless | 8.63 ± 0.01 | 8.34 ± 0.01 | 3.48 |
| 0.10 M Na2CO3 | Colorless | 11.12 ± 0.06 | 11.37 ± 0.01 | 2.20 |
| Commercial Dettol Detergent | Transparent Brown | 9.58 ± 0.03 | 9.68 ± 0.02 | 1.03 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Thabit, N.; Umar, Y.; Ratemi, E.; Ahmad, A.; Ahmad Abuilaiwi, F. A Flexible Optical pH Sensor Based on Polysulfone Membranes Coated with pH-Responsive Polyaniline Nanofibers. Sensors 2016, 16, 986. https://doi.org/10.3390/s16070986
Abu-Thabit N, Umar Y, Ratemi E, Ahmad A, Ahmad Abuilaiwi F. A Flexible Optical pH Sensor Based on Polysulfone Membranes Coated with pH-Responsive Polyaniline Nanofibers. Sensors. 2016; 16(7):986. https://doi.org/10.3390/s16070986
Chicago/Turabian StyleAbu-Thabit, Nedal, Yunusa Umar, Elaref Ratemi, Ayman Ahmad, and Faraj Ahmad Abuilaiwi. 2016. "A Flexible Optical pH Sensor Based on Polysulfone Membranes Coated with pH-Responsive Polyaniline Nanofibers" Sensors 16, no. 7: 986. https://doi.org/10.3390/s16070986
APA StyleAbu-Thabit, N., Umar, Y., Ratemi, E., Ahmad, A., & Ahmad Abuilaiwi, F. (2016). A Flexible Optical pH Sensor Based on Polysulfone Membranes Coated with pH-Responsive Polyaniline Nanofibers. Sensors, 16(7), 986. https://doi.org/10.3390/s16070986