Silicon Nanocrystals with pH-Sensitive Tunable Light Emission from Violet to Blue-Green
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Morphology Characterization and Size Statistics
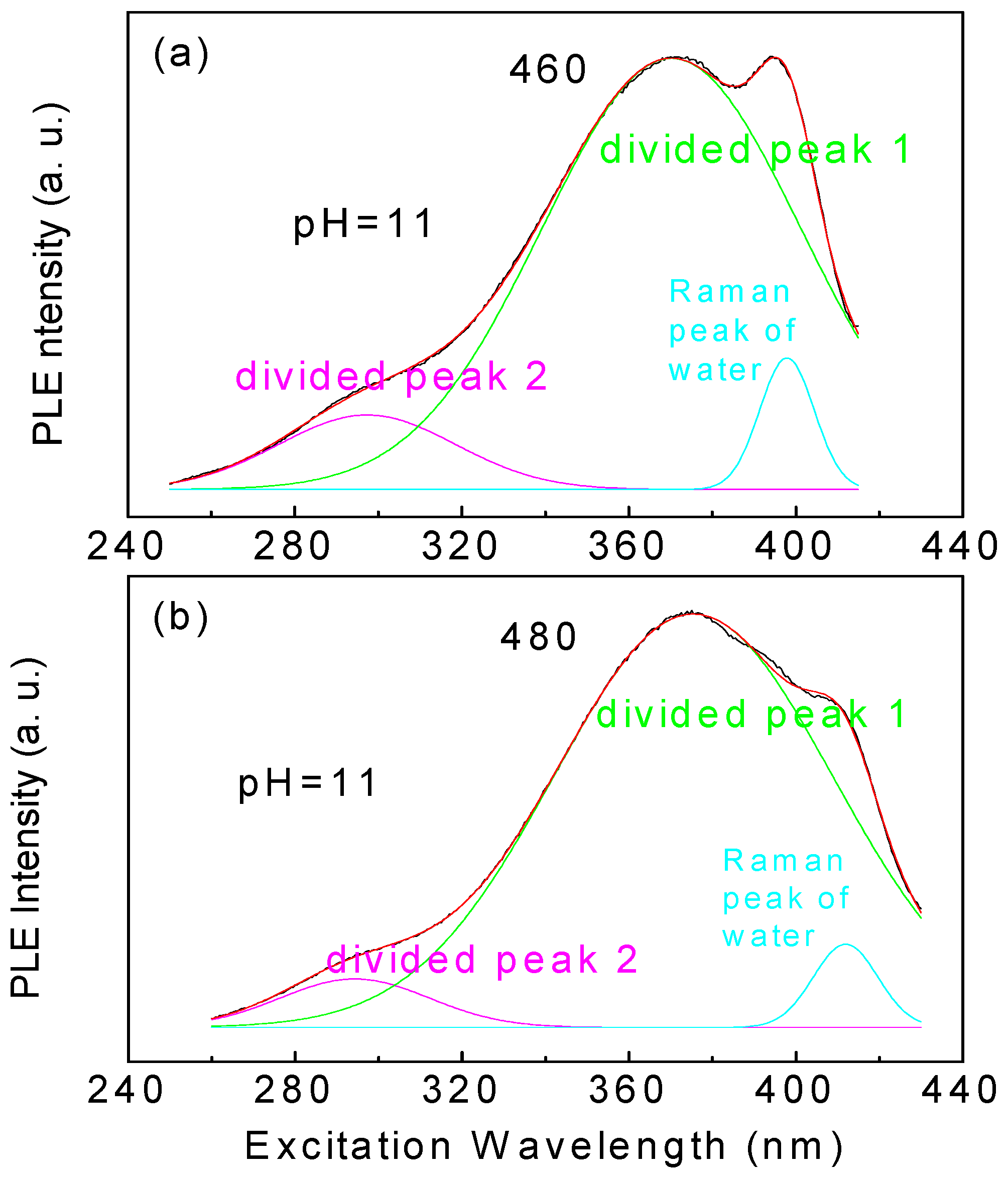
3.2. PL and PLE Spectral Characteristic
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Kim, U.; Kim, I.; Park, Y.; Lee, K.Y.; Yim, S.Y.; Park, J.G.; Ahn, H.G.; Park, S.H.; Choi, H.J. Synthesis of Si Nanosheets by a Chemical Vapor Deposition Process and Their Blue Emissions. ACS Nano 2011, 5, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Guo, L.; Chou, S.Y. Silicon single-electron quantum-dot transistor switch operating at room temperature. Appl. Phys. Lett. 1998, 72, 1205–1207. [Google Scholar] [CrossRef]
- Cui, Y.; Lieber, C.M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-temperature ultraviolet nanowire nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Erogbogbo, F.; Yong, K.T.; Roy, I.; Xu, G.; Prasad, P.N.; Swihart, M.T. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano 2008, 2, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Wolkin, M.V.; Jorne, J.; Fauchet, P.M. Electronic states and luminescence in porous silicon quantum dots: The role of oxygen. Phys. Rev. Lett. 1999, 82, 197–200. [Google Scholar] [CrossRef]
- Cullis, A.G.; Canham, L.T.; Calcott, P.D.J. The structural and luminescence properties of porous silicon. J. Appl. Phys. 1997, 82, 909–965. [Google Scholar] [CrossRef]
- Canham, L.T. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 1990, 57 (Suppl. 10), 1046–1048. [Google Scholar] [CrossRef]
- Hao, X.J.; Podhorodecki, A.P.; Shen, Y.S.; Zatryb, G.; Misiewicz, J.; Green, M.A. Effects of Si-rich oxide layer stoichiometry on the structural and optical properties of Si QD/SiO2 multilayer films. Nanotechnology 2009, 20, 485703–485712. [Google Scholar] [CrossRef] [PubMed]
- Holunga, D.M.; Flagan, R.C.; Atwater, H.A. A scalable turbulent mixing aerosol reactor for oxide-coated silicon nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 6332–6341. [Google Scholar] [CrossRef]
- Littau, K.A.; Szajowski, P.J.; Muller, A.J.; Kortan, A.R.; Brus, L.E. A luminescent silicon nanocrystal colloid via a high-temperature aerosol reaction. J. Phys. Chem. 1993, 97, 1224–1230. [Google Scholar] [CrossRef]
- Lopez, J.A.L.; Roman, A.G.; Barojas, E.G.; Gracia, J.F.F.; Juarez, J.M.; Lopez, J.C. Synthesis of colloidal solutions with silicon nanocrystals from porous silicon. Nanoscale Res. Lett. 2014, 9, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Valenta, J.; Jenada, P.; Dohnalová, K.; Niznansky, D.; Vácha, F.; Linnros, J. Colloidal suspensions of silicon nanocrystals: From single nanocrystals to photonic structures. Opt. Mater. 2005, 27 (Suppl. 5), 1046–1049. [Google Scholar] [CrossRef]
- Tilley, R.D.; Warner, J.H.; Yamamoto, K.; Matsui, I.; Fujimori, H. Micro-emulsion synthesis of monodisperse surface stabilized silicon nanocrystals. Chem. Commun. 2005, 14, 1833–1835. [Google Scholar] [CrossRef] [PubMed]
- Umezu, I.; Minami, H.; Senoo, H.; Sugimura, A. Synthesis of photoluminescent colloidal silicon nanoparticles by pulsed laser ablation in liquids. J. Phys. 2007, 59 (Suppl. 1), 392–395. [Google Scholar] [CrossRef]
- Bagabas, A.A.; Gondal, M.A.; Dastageer, M.A.; Al-Muhanna, A.A.; Alanazi, T.H.; Ababtain, M.A. A study of laser-induced blue emission with nanosecond decay of silicon nanoparticles synthesized by a chemical etching method. Nanotechnology 2009, 20, 355703. [Google Scholar] [CrossRef] [PubMed]
- Yixuan, Y.; Clare, E.R.; Richard, D.S.; Brian, A.K. Synthesis and Ligand Exchange of Thiol-capped Silicon Nanocrystals. Langmuir 2015, 31, 6886–6893. [Google Scholar] [CrossRef]
- Ondic, L.; Varga, M.; Pelant, I.; Valenta, J.; Kromka, A.; Elliman, R.G. Silicon nanocrystal-based photonic crystal slabs with broadband and efficient directional light emission. Sci. Rep. 2017, 7, 5763. [Google Scholar] [CrossRef] [PubMed]
- Svrcek, V.; McDonald, C.; Lozac’h, M.; Tayagaki, T.; Koganezawa, T.; Miyadera, T.; Mariotti, D.; Matsubara, K. Stable ultrathin surfactant-free surface-engineered silicon nanocrystal solar cells deposited at room temperature. Energy Sci. Eng. 2017, 5, 184–193. [Google Scholar] [CrossRef]
- Cheng, K.; Anthony, R.; Kortshagen, U.; Holmes, R. High-Efficiency Silicon Nanocrystal Light-Emitting Devices. Nano Lett. 2011, 11, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, B.; Brenny, B.J.M.; Dekker, S.; Dogan, I.; Schall, P.; Dohnalova, K. Multi-chromatic silicon nanocrystals. Light Sci. Appl. 2017, 6, e17007. [Google Scholar] [CrossRef]
- Chaabane, N.; Cabarrocas, P.R.; Vach, H. Trapping of plasma produced nanocrystalline Si particles on a low temperature substrate. J. Non-Cryst. Solids 2004, 338, 51–55. [Google Scholar] [CrossRef]
- Nayfeh, M.H.; Rao, S.; Nayfeh, O.M.; Smith, A.; Therrien, J. UV photodetectors with thin-film Si nanoparticle active medium. IEEE Trans. Nanotechnol. 2005, 4 (Suppl. 6), 660–668. [Google Scholar] [CrossRef]
- Erogbogbo, F.; Yong, K.T.; Hu, R.; Law, W.C.; Ding, H.; Chang, C.W.; Prasad, P.N.; Swihart, M.T. Biocompatible Magnetofluorescent Probes: Luminescent Silicon Quantum Dots Coupled with Superparamagnetic Iron(III) Oxide. ACS Nano 2010, 4, 5131–5138. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. New approaches for medicinal applications of bioinorganic chemistry. Curr. Opin. Chem. Biol. 2007, 11, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Beard, M.C. Multiple Exciton Generation in Semiconductor Quantum Dots. J. Phys. Chem. Lett. 2011, 2, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Izeddin, I.; Stallinga, P.; Yassievich, I.N.; Gregorkiewicz, T. Space-separated quantum cutting with silicon nanocrystals for photovoltaic applications. Nat. Photonics 2008, 2, 105–109. [Google Scholar] [CrossRef]
- Yoffe, A.D. Low-dimensional systems: Quantum size effects and electronic properties of semiconductor microcrystallites (zero-dimensional systems) and some quasi-two-dimensional systems. Adv. Phys. 2002, 51, 799–890. [Google Scholar] [CrossRef]
- Fuechsle, M.; Mahapatra, S.; Zwanenburg, F.; Friesen, M.; Eriksson, M.; Simmons, M. Spectroscopy of few-electron single-crystal silicon quantum dots. Nat. Nanotechnol. 2010, 5, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Bagolini, L.; Mattoni, A.; Fugallo, G.; Colomb, L. Quantum Confinement by an Order-Disorder Boundary in Nanocrystalline Silicon. Phys. Rev. Lett. 2010, 104, 176803. [Google Scholar] [CrossRef] [PubMed]
- Beard, M.; Knutsen, K.P.; Yu, P.R.; Luther, J.M.; Song, Q.; Metzger, W.K.; Ellingson, R.J.; Nozik, A.J. Multiple exciton generation in colloidal silicon nanocrystals. Nano Lett. 2007, 7, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Cibulka, O.; Vorkotter, C.; Purkrt, A.; Holovsky, J.; Benedikt, J.; Herynkova, K. Comparison of Silicon Nanocrystals Prepared by Two Fundamentally Different Methods. Nanoscale Res. Lett. 2016, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.C.; Tang, L.; Huang, J.D.; Voutsas, A.; Lin, L.Y. Visible electroluminescence from hybrid colloidal silicon quantum dot-organic light-emitting diodes. Appl. Phys. Lett. 2011, 98, 213102. [Google Scholar] [CrossRef]
- Gupta, A.; Swihart, M.; Wiggers, H. Luminescent Colloidal Dispersion of Silicon Quantum Dots from Microwave Plasma Synthesis: Exploring the Photoluminescence Behavior Across the Visible Spectrum. Adv. Funct. Mater. 2009, 19, 696–703. [Google Scholar] [CrossRef]
- Godefroo, S.; Hayne, M.; Jivanescu, M.; Stesmans, A.; Zacharias, M.; Lebedev, O.; Van Tendeloo, G.; Moshchalkov, V. Classification and control of the origin of photoluminescence from Si nanocrystals. Nat. Nanotechnol. 2008, 3, 174–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.Y.; Lu, Y.F.; Wu, Y.H.; Cho, B.J.; Liu, M.H.; Dai, D.Y.; Song, W.D. Mechanisms of photoluminescence from silicon nanocrystals formed by pulsed-laser deposition in argon and oxygen ambient. J. Appl. Phys. 2003, 93, 6311–6319. [Google Scholar] [CrossRef]
- Schmidt, J.U.; Schmidt, B. Investigation of Si nanocluster formation in sputter-deposited silicon sub-oxides for nanocluster memory structures. Mater. Sci. Eng. 2003, 101, 28–33. [Google Scholar] [CrossRef]
- Kusova, K.; Cibulka, O.; Dohnalova, K.; Pelant, I.; Valenta, J.; Fucikova, A.; Zidek, K.; Lang, J.; Englich, J.; Matejka, P.; et al. Brightly Luminescent Organically Capped Silicon Nanocrystals Fabricated at Room Temperature and Atmospheric Pressure. ACS Nano 2010, 4, 4495–4504. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Nakamura, T.; Adachi, S.; Matsuishi, K. Improvement of Laser Processing for Colloidal Silicon Nanocrystal Formation in a Reactive Solvent. J. Phys. Chem. Lett. 2017, 121, 8623–8629. [Google Scholar] [CrossRef]
- Nielsen, D.; Abdulhassan, L.; Alchihabi, M.; Al-Muhanna, A.; Host, J.; Nayfah, M.H. Current-less anodization of intrinsic silicon powder grains: Formation of fluorescent Si nanoparticles. J. Appl. Phys. 2007, 101, 114302. [Google Scholar] [CrossRef]
- Nozaki, T.; Sasaki, K.; Ogino, T.; Asahi, D.; Okazaki, K. Microplasma synthesis of tunable photoluminescent silicon nanocrystals. Nanotechnology 2007, 18, 235603. [Google Scholar] [CrossRef]
- Svrek, V.; Mariotti, D.; Kondo, M. Microplasma-induced surface engineering of silicon nanocrystals in colloidal dispersion. Appl. Phys. Lett. 2010, 97, 161502. [Google Scholar] [CrossRef]
- Zhang, Q.; Bayliss, S.C.; Hutt, D.A. Blue photoluminescence and local-structure of Si nanostructures embedded in SiO2 matrices. Appl. Phys. Lett. 1995, 66, 1977–1979. [Google Scholar] [CrossRef]
- Ray, M.; Hossain, S.M.; Klie, R.F.; Banerjee, K.; Ghosh, S. Free standing luminescent silicon quantum dots: evidence of quantum confinement and defect related transitions. Nanotechnology 2010, 21 (Suppl. 50), 505602. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.Z.; Wakimoto, R.; Saitow, K. Synthesis of Size-controlled Luminescent Si Nanocrystals from (HSiO1.5)(n) Polymers. Chem. Lett. 2017, 46, 699–702. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.L.; Li, T.H.; Chu, P.K. All-silicon solid films with highly efficient and tunable full-color photoluminescence. Scripta Mater. 2014, 76, 17–20. [Google Scholar] [CrossRef]
- Vanhellemont, J.; De Gryse, O.; Clauws, P. Critical precipitate size revisited and implications for oxygen precipitation in silicon. Appl. Phys. Lett. 2005, 86, 221903. [Google Scholar] [CrossRef]
- Heintz, A.; Fink, M.; Mitchell, B. Mechanochemical synthesis of blue luminescent alkyl/alkenyl-passivated silicon nanoparticles. Adv. Mater. 2007, 19, 3984. [Google Scholar] [CrossRef]
- Podhorodecki, A.; Misiewicz, J.; Gourbilleau, F.; Rizk, R. Absorption mechanisms of silicon nanocrystals in cosputtered silicon-rich-silicon oxide films. Electrochem. Solid-State Lett. 2008, 11, K31–K33. [Google Scholar] [CrossRef]
- Zhou, Z.; Brus, L.; Friesner, R. Electronic structure and luminescence of1.1- and 1.4-nm silicon nanocrystals: Oxide shell versus hydrogenpassivation. Nano Lett. 2003, 3, 163–167. [Google Scholar] [CrossRef]
- Kujala, J.; Slotte, J.; Tuomisto, F.; Hiller, D.; Zacharias, M. Si nanocrystals and nanocrystal interfaces studied by positron annihilation. J. Appl. Phys. 2016, 120, 145302. [Google Scholar] [CrossRef]
- Li, H.P.; Xu, H.; Shen, X.P.; Han, K.; Bi, Z.T.; Xu, R.F. Size-, electric- field-, and frequency-dependent third-order nonlinear optical properties of hydrogenated silicon nanoclusters. Sci. Rep. 2016, 6, 28067. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.K.; Liao, K.; Casillas, G.; Li, Y.Y.; Ozin, G.A. Cationic Silicon Nanocrystals with Colloidal Stability, pH-Independent Positive Surface Charge and Size Tunable Photoluminescence in the Near-Infrared to Red Spectral Range. Adv. Sci. 2016, 3, 1500263. [Google Scholar] [CrossRef] [PubMed]
- Botas, A.M.P.; Anthony, R.J.; Wu, J.; Rowe, D.J.; Silva, N.J.O.; Kortshagen, U.; Pereira, R.N.; Ferreira, R.A.S. Influence of the surface termination on the light emission of crystalline silicon nanoparticles. Nanotechnology 2016, 27, 325703. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hessel, C.M.; Bogart, T.D.; Panthani, M.G.; Rasch, M.R.; Korgel, B. Room Temperature Hydrosilylation of Silicon Nanocrystals with Bifunctional Terminal Alkenes. Langmuir 2013, 29, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Ghosh, B.; Beaune, G.; Nagarajan, U.; Yasui, T.; Nakamura, J.; Tsuruoka, T.; Baba, Y.; Shirahata, N.; Winnik, F.M. Functional double-shelled silicon nanocrystals for two-photon fluorescence cell imaging: Spectral evolution and tuning. Nanoscale 2016, 8, 9009–9019. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Sugimoto, H.; Imakita, K. All-inorganic colloidal silicon nanocrystals-surface modification by boron and phosphorus co-doping. Nanotechnology 2016, 27, 262001. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qian, C.X.; Chen, K.K.; Ozin, G.A. Silicon Nanocrystals: It’s Simply a Matter of Size. Chemnanomat 2016, 2, 847–855. [Google Scholar] [CrossRef]
- Han, P.G.; Poon, M.C.; Sin, K.O.; Wong, M. Photoluminescent porous polycrystalline silicon. In Proceedings of the Electron Devices Meeting, Clearwater Bay, Hong Kong, China, 1 July 1995. [Google Scholar]
- Maxfield, F.R.; McGraw, T.E. Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 2004, 5, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Yuli, I.; Oplatka, A. Cytosolic acidification as an early transductory signal of human neutrophil chemotaxis. Science 1987, 235, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Ritchie, K.; Kasai, R.S.; Morone, N.; Sugimura, H.; Tanaka, K.; Sase, I.; Yoshimura, A.; Nakano, Y.; Fujiwara, T.K.; et al. Biocompatible fluorescent silicon nanocrystals for single-molecule tracking and fluorescence imaging. J. Cell Biol. 2013, 202, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Cohen-Kami, T.; Qing, Q.; Duan, X.; Xie, P.; Lieber, C. Three-Dimensional, Flexible Nanoscale Field-Effect Transistors as Localized Bioprobes. Science 2010, 329, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tapec-Dytioco, R.; Tan, W. Ultrasensitive DNA detection using highly fluorescent bioconjugated nanoparticles. J. Am. Chem. Soc. 2003, 125, 11474–11475. [Google Scholar] [CrossRef] [PubMed]





| PL Peak Wavelength | pH = 1 | pH = 3 | pH = 5 | pH = 7 | pH = 9 | pH = 11 |
|---|---|---|---|---|---|---|
| 320 | 381 | 390 | 400 | 405 | 402 | 410 |
| 340 | 400 | 407 | 412 | 420 | 424 | 429 |
| 350 | - | - | 414 | 425 | - | - |
| 360 | 430 | 433 | 436 | - | 443 | 447 |
| 370 | - | - | 450 | 450 | 450 | - |
| 380 | 455 | 460 | - | - | - | - |
| 390 | - | - | 460 | 464 | 462 | 464 |
| pH Value | Emission Wavelength (nm) | Center Wavelength of Divided Peak 1 | Center Wavelength of Divided Peak 2 |
|---|---|---|---|
| 1 | 450 | 349 | N/A |
| 3 | 480 | 355 | 405 |
| 5 | 470 | 358 | 343 |
| 7 | 470 | 361 | 322 |
| 9 | 480 | 375 | 297 |
| 11 | 470 | 373 | 293 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Guo, J.; Chen, J. Silicon Nanocrystals with pH-Sensitive Tunable Light Emission from Violet to Blue-Green. Sensors 2017, 17, 2396. https://doi.org/10.3390/s17102396
Wang J, Guo J, Chen J. Silicon Nanocrystals with pH-Sensitive Tunable Light Emission from Violet to Blue-Green. Sensors. 2017; 17(10):2396. https://doi.org/10.3390/s17102396
Chicago/Turabian StyleWang, Jing, Junhong Guo, and Jing Chen. 2017. "Silicon Nanocrystals with pH-Sensitive Tunable Light Emission from Violet to Blue-Green" Sensors 17, no. 10: 2396. https://doi.org/10.3390/s17102396




