Adsorptive Stripping Voltammetric Determination of Amaranth and Tartrazine in Drinks and Gelatins Using a Screen-Printed Carbon Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Chemicals and Reagents
2.3. Cyclic and Square Wave Voltammograms
3. Results and Discussion
3.1. Electrochemical Behavior of AM and TZ on SPCE as a Function of pH
3.2. Effect of Supporting Electrolyte
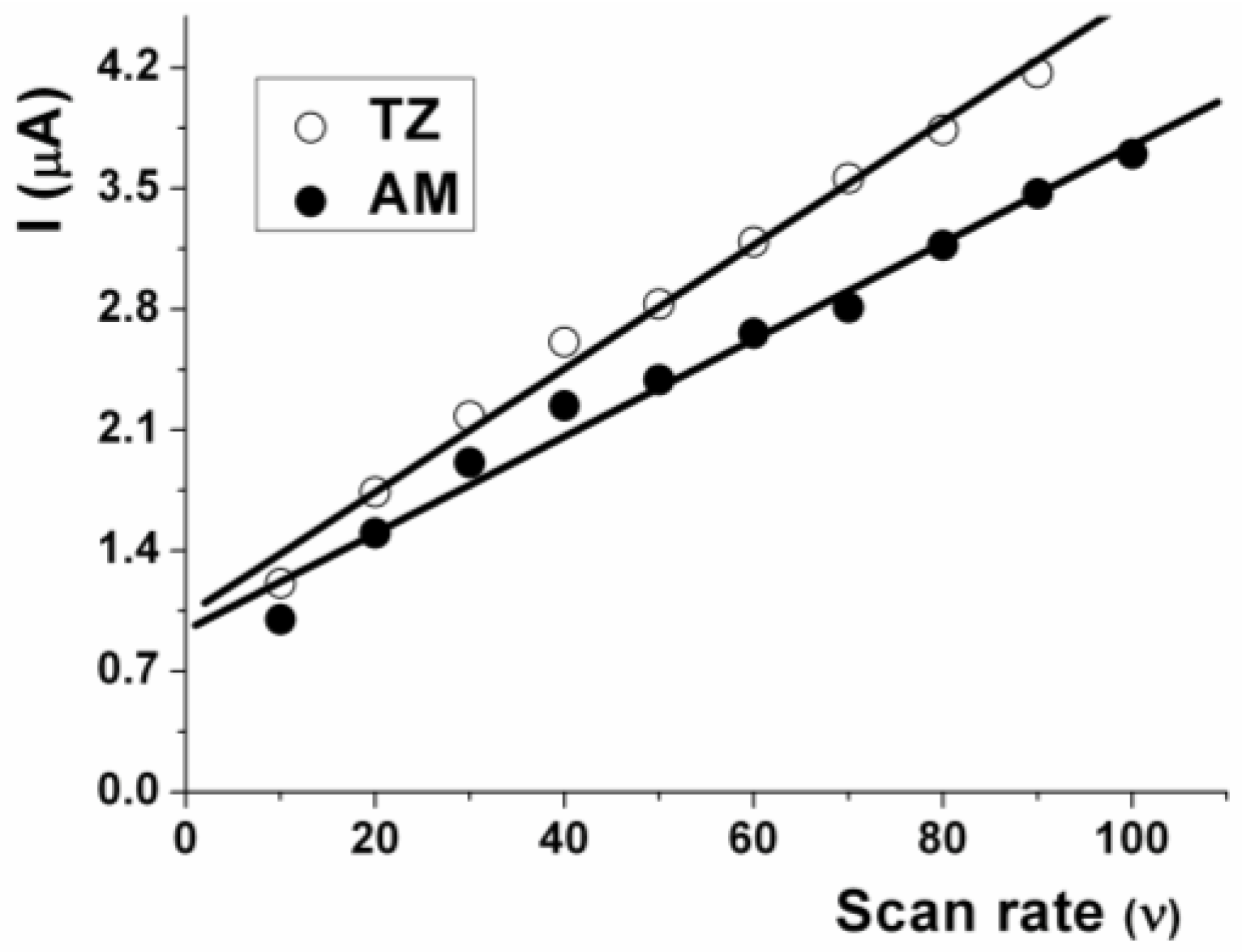
3.3. Influence of the Scan Rate (υ)
3.4. Effect of Adsorption Potential and Time (Eads, tads) on the Accumulation Step
3.5. Effect of Instrumental Variables (Frequency, Step Potential, and Amplitude)
3.6. Linear Range, Detection Limit, and Repeatability of the Method
3.7. Validation of the Method and Interference Studies
3.8. Application to Real Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mpountoukas, P.; Pantazaki, A.; Kostareli, E.; Christodoulou, P.; Kareli, D.; Poliliou, S.; Mourelatos, C.; Lambropoulou, V.; Lialiaris, T. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem. Toxicol. 2010, 48, 2934–2944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ma, Y. Mechanistic and conformational studies on the interaction of food dye amaranth with human serum albumin by multispectroscopic methods. Food Chem. 2013, 136, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T. Mutagenicity and carcinogenicity of aromatic amines metabolically produced from azo dyes. J. Environ. Sci. Heal C 2000, 18, 51–74. [Google Scholar] [CrossRef]
- Rovina, K.; Siddiquee, S.; Shaarani, S. Toxicology, extraction and analytical methods for determination of amaranth in food and beverage products. Trends Food Sci. Technol. 2017, 65, 68–79. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, J.B.; Du, L.M.; Tian, H.; Hao, C.X.; Wang, Z.F.; Wang, J.Y. A rapid shaking-based ionic liquid dispersive liquid phase microextraction for the simultaneous determination of six synthetic food colourants in soft drinks, sugar-and gelatin-based confectionery by high-performance liquid chromatography. Food Chem. 2013, 141, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Luo, X.; Chen, B.; Su, S.; Yao, S. Simultaneous determination of water-soluble and fat-soluble synthetic colorants in foodstuff by high-performance liquid chromatography–diode array detection–electrospray mass spectrometry. J. Chromatogr. A 2006, 1103, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Bai, J.; Jin, L. Multicomponent chemometric determination of colorant mixtures by Voltammetry. Anal. Lett. 1997, 30, 1761–1777. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Brooks, J.; Hart, J.P. Development of a voltammetric assay, using screen-printed electrodes, for clonazepam and its application to beverage and serum samples. Talanta 2016, 147, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Arancibia, V.; Rojas, C.; Nagles, E. Adsorptive stripping voltammetric determination of tartrazine and sunset yellow in gelatins and soft drink powder in the presence of cetylpyridinium bromide. Int. J. Electrochem. Sci. 2012, 7, 7493–7502. [Google Scholar]
- Wang, M.; Gao, Y.; Sun, Q.; Zhao, J. Ultrasensitive and simultaneous determination of the isomers of amaranth and ponceau 4R in foods based on new carbon nanotube/polypyrrole composites. Food Chem. 2015, 172, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, M.; Shentu, C.; Wang, W.; Chen, Z. Simultaneous determination of the isomers of ponceau 4R and amaranth using an expanded graphite paste electrode. Food Chem. 2014, 160, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, Y.; Yang, X.; Zhao, J. Sensitive determination of amaranth in drinks by highly dispersed CNT in graphene oxide ‘‘water’’ with the aid of small amounts of ionic liquid. Food Chem. 2015, 179, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, K.; Nagles, E.; Arancibia, V.; Otiniano, M. Optimizing adsorption voltammetric technique (AdSV) in determining of amaranth on carbon printed electrodes. Effect of surfactants on sensitivity. Rev. Soc. Quím. Perú 2014, 80, 115–123. [Google Scholar]
- Alghamdi, A.H. A square-wave adsorptive stripping voltammetric method for the determination of amaranth, a food additive dye. J. AOAC Int. 2005, 88, 788–793. [Google Scholar] [PubMed]
- Yu, L.; Zheng, H.; Shi, M.; Jing, S.; Qu, L. A Novel Electrochemical Sensor Based on Poly (Diallyldimethylammonium Chloride)-Dispersed Graphene Supported Palladium Nanoparticles for Simultaneous Determination of Sunset Yellow and Tartrazine in Soft Drinks. Food Anal. Methods 2017, 10, 200–209. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous determination of sunset yellow and tartrazine in soft drinks using gold nanoparticles carbon paste electrode. Food Chem. 2012, 132, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Sun, J.; Cao, S.; Gao, F.; Zhang, Y.; Yang, Y. One-step electrochemical approach for the preparation of grapheme wrapped-phosphotungstic acid hybrid and its application for simultaneous determination of sunset yellow and tartrazine. Electrochim. Acta 2012, 74, 151–157. [Google Scholar] [CrossRef]
- Medeiros, R.A.; Lourencao, B.C.; Rocha-Filho, R.C.; Fatibello-Filho, O. Simultaneous voltammetric determination of synthetic colorants in food using a cathodically pretreated boron-doped diamond electrode. Talanta 2012, 97, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Sun, J.; Meng, W.; Song, L.; Zhang, Y. Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food. Food Chem. 2013, 141, 3731–3737. [Google Scholar] [CrossRef] [PubMed]
- Jampasa, S.; Siangproh, W.; Duangmal, K.; Chailapakul, O. Electrochemically reduced graphene oxide-modified screen-printed carbon electrodes for a simple and highly sensitive electrochemical detection of synthetic colorants in beverages. Talanta 2016, 160, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Becerro-Dominguez, F.; Gonzalez-Diego, F.; Hernandez-Mendez, J. Determination of sunset yellow and tartrazine by differential pulse polarography. Talanta 1990, 37, 655–658. [Google Scholar] [CrossRef]
- Lipskikh, O.I.; Nikolaeva, A.A.; Korotkova, E.I. Voltammetric determination of tartrazine in food. J. Anal. Chem. 2017, 72, 396–401. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Zuo, J.; Zhang, J.; Zhu, L.; Zhang, J. Rapid and sensitive determination of tartrazine using a molecularly imprinted copolymer modified carbon electrode (MIP-PmDB/PoPD-GCE). J. Electroanal. Chem. 2017, 785, 90–95. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Wu, Q.; Jing, Q.; Yu, S. Graphene decorated with nickel nanoparticles as a sensitive substrate for simultaneous determination of sunset yellow and tartrazine in food samples. Electroanalysis 2013, 25, 1505–1512. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Lingane, J. Polarography; Interscience Publishers: New York, NY, USA, 1952; Volume 2, p. 768. [Google Scholar]





| Dye | Electrode | Method | Recovery (%) | DL (µmol L−1) | Samples | Ref. |
|---|---|---|---|---|---|---|
| Amaranth Tartrazine | HMDE | reduction | --- --- | Foods | [8] | |
| Tartrazine | HMDE | reduction | 3.30 µg/L | Flavored gelatin | [11] | |
| Amaranth | CNT–ppy-GCE | oxidation | 93.0 | 0.0005 | Fruit drinks | [12] |
| Amaranth | EGPE | oxidation | 98.0 | 0.036 | Grape juice | [13] |
| Amaranth | CNT/GO-IL-GCE | oxidation | 95.0–105.0 | 0.0001 | Foods | [14] |
| Amaranth | SPCE | oxidation | 0.018 | Soft drinks | [15] | |
| Amaranth | HMDE | reduction | 104.0 | 0.0017 | Soft drinks | [16] |
| Amaranth | PDDA-Gr-Pd/GCE | oxidation | 0.005 | Soft drinks | [17] | |
| Tartrazine | nAu-CPE | oxidation | 96.0–104.0 | 0.002 | Soft drinks | [18] |
| Tartrazine | GN–PTA-GCE | oxidation | 95.0–104.0 | 30 µg/L | Soft drinks | [19] |
| Tartrazine | BDDE | oxidation | 95.0 | 0.0627 | Foods | [20] |
| Tartrazine | GN/TiO2-CPE | oxidation | 99.0–102.0 | 0.008 | Foods | [21] |
| Tartrazine | ERGO-SPCE | oxidation | 0.0045 | Foods | [22] | |
| Tartrazine | HMDE | reduction | 0.03 | Soft drinks | [23] | |
| Tartrazine | GCE | reduction | 0.011 mg/L | Foods | [24] | |
| Tartrazine | MIP-PmDB/PoPD-GCE | oxidation | 0.0035 | Soft drinks | [25] | |
| Tartrazine | GN-Ni/GCE | oxidation | 0.00108 | Foods | [26] |
| Unflavored Gelatin | Added (µmol L−1) | Found (µmol L−1) | % R. Error | |||
|---|---|---|---|---|---|---|
| samples | AM | TZ | AM | TZ | AM | TZ |
| 1 | 3.13 | 7.0 | 2.53 | 8.08 | −19.0 | 15.4 |
| 2 | 1.61 | 1.68 | 2.00 | 1.31 | 24.2 | −22.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdomo, Y.; Arancibia, V.; García-Beltrán, O.; Nagles, E. Adsorptive Stripping Voltammetric Determination of Amaranth and Tartrazine in Drinks and Gelatins Using a Screen-Printed Carbon Electrode. Sensors 2017, 17, 2665. https://doi.org/10.3390/s17112665
Perdomo Y, Arancibia V, García-Beltrán O, Nagles E. Adsorptive Stripping Voltammetric Determination of Amaranth and Tartrazine in Drinks and Gelatins Using a Screen-Printed Carbon Electrode. Sensors. 2017; 17(11):2665. https://doi.org/10.3390/s17112665
Chicago/Turabian StylePerdomo, Yeny, Verónica Arancibia, Olimpo García-Beltrán, and Edgar Nagles. 2017. "Adsorptive Stripping Voltammetric Determination of Amaranth and Tartrazine in Drinks and Gelatins Using a Screen-Printed Carbon Electrode" Sensors 17, no. 11: 2665. https://doi.org/10.3390/s17112665






