1. Introduction
The carbon cycle in the ocean is a dynamic component of the global carbon budget, but the diverse sources and carbon sinks as well as their complex interactions in the ocean remain poorly understood [
1,
2]. The dissolved inorganic carbon (DIC) in the ocean is the sum of HCO
3−, CO
32−, H
2CO
3 and CO
2 [
3]. The concentration of DIC is about 2.30 mmol/L, with
(about 1.95 mmol/L) making up the majority of it [
4]. Therefore, bicarbonate is a key factor in carbon cycle research. The modern methods for oceanic carbonate system measurement are mainly involved in total carbon and CO
2 partial pressure (pCO
2) investigations [
5,
6]. However, the direct detection of HCO
3− and CO
32− in seawater it is still a huge challenge due to their low concentrations. Raman spectroscopy is potentially capable of overcoming this challenge on the condition of a significant ensitivity enhancement [
7].
In 2004, scientists reported the first attempt to detect HCO
3− in water solutions directly by using Raman spectroscopy [
8], with a limit of detection (LOD) of 7.5 mmol/L after an integration time of 20 min. They had also tried to enhance the HCO
3− signal with an introduced liquid core waveguide [
9], eventually obtaining an amplification ratio of 7.8× in the peak intensity. All those efforts still do not make the direct Raman detection of HCO
3− in seawater practical due to the fact its concentration is as low as 2 mmol/L. The strong background signal resulting from SO
42−, which has a concentration of ~28 mmol/L in seawater [
10], makes direct detection of HCO
3− even more difficult. With a similar waveguide-based enhancement mechanism, the multi-pass cavity concept has been presented as an effective approach in gas Raman detection with desirable enhancement [
11,
12,
13,
14]. Taking such an approach in direct seawater Raman detection is the motivation for the work presented in this article. A near-concentric cavity-enhanced Raman spectroscopy system was specially built for liquid sample detection. With the aid of this system, direct HCO
3− Raman detection in seawater becomes readily feasible as expected.
2. Experiment Setup
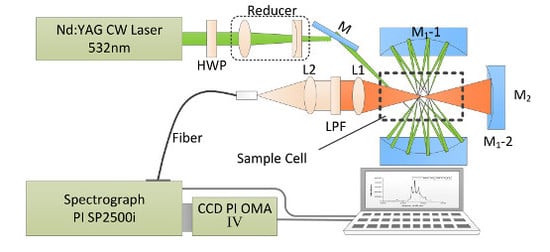
As shown in
Figure 1, the near-concentric cavity-enhanced Raman spectroscopy system is adapted on the basis of a formerly reported design [
14]. In order to detect liquid samples, a sample cell of fused silica with 10 mm × 10 mm × 40 mm inner size and 1 mm wall thickness is used and placed at the center of the cavity. In this system, a diode-pumped, frequency-doubled Nd:YAG CW laser (532 nm) with a power of 300 mW is used as the Raman excitation laser source. The polarization of the laser beam is rotated by 90° through passing a half wave plate (HWP), and then the laser beam is compressed and focused into the chamber by a couple of lens (a planoconvex lens with a focal length of 100 mm and a planoconcave lens with a focal length of −75 mm). The near concentric cavity is composed of two identical spherical mirrors M1 with 25.4 mm diameter and 25 mm focal length. The reflectivity of these two mirrors is over 99% from 500 to 700 nm. These two mirrors are spaced face to face at a distance of 104.2 mm, and the mirror M1-2 is clockwise rotated about 0.04° to create the near concentric cavity reflection mode. Because of the strong absorption of water, the laser beam number in the cavity is 18, which is less than the number in the cavity of a gas sample cell. The scattering signal from the center of cavity is collected by an achromatic doublet lens L1 (f = 30 mm) with a diameter of 25.4 mm. A mirror M2 (f = 12.5 mm), placed on the opposite side beyond the sample chamber, is used to enhance the collection efficiency of the signal. Another lens L2 (f = 30 mm) is used to couple the Raman signal into the delivery optical fiber bundle (19 μm × 100 μm, NA = 0.22), with a long pass filter placed on its front to get rid of Rayleigh scattering from the collected signal. From the other end of the fiber, the signal is then coupled into a spectrometer (Acton SP2500i, Princeton Instruments, Trenton, NJ, USA), with 1200 g/mm grating and 10 μm entrance slit width. The Raman spectra are recorded by a CCD detector (SPEC-10: 400B, Princeton Instruments, Trenton, NJ, USA) with a 1340 × 400 imaging array and 20 μm × 20 μm pixel size operating at −40 °C. The resulted spectral range is 800~2000 cm
−1 with a resolution of 2 cm
−1. To get a relative desirable Raman signal and prevent the CCD from overexposure, each spectrum is accumulated five times with an integration time of 10 s, so the total acquisition time is 50 s for one sample. Five measurements are taken for each sample to evaluate the repeatability.
3. Results
The reported LOD for SO
42− by using a liquid core optical fiber Raman system is about 1.5 mmol/L [
15]. As a comparison, we prepared 15 Na
2SO
4 solutions (0.10 mmol/L–2.00 mmol/L) to evaluate the LOD of the NC-CERS system. The LOD is defined as the ratio of three times the noise intensity (σ) to the slope of calculation curve. The spectra of four solutions are shown in
Figure 2a, and the Raman signal of SO
42− can be observed clearly in the spectrum of the 0.10 mmol/L solution. Given this, the NC-CERS system has a LOD less than 0.10 mmol/L for SO
42− which is 15 times lower than that reported before. The linear relationships between concentrations and peak intensity of the SO
42− are shown in
Figure 2b and the response of the NC-CERS system for liquid detection has good linearity with R
2 = 0.996 over the whole range.
To evaluate its performance for HCO
3−, we prepared nine NaHCO
3 solutions with concentrations ranging from 0.40 mmol/L to 4.00 mmol/L. The corresponding concentrations of HCO
3− are from 0.37 mmol/L to 3.79 mmol/L calculated by using the carbon balance mode. All of the concentrations of HCO
3− are corrected by using the equilibrium mode of carbon components in water.
Figure 3a shows the spectra of five of these concentrations. The peak intensity of the HCO
3− Raman signal increases as a function of the concentration, and the Raman signal of HCO
3− can be distinguished in the solution with 0.37 mmol/L NaHCO
3. It is proved that the LOD of the NC-CERS system for HCO
3− is less than 0.37 mmol/L, which is much lower than its concentration in seawater. The linear relationship between concentrations and peak intensities of the HCO
3− is shown in
Figure 3b and it shows good linearity with R
2 = 0.978.
Furthermore, we prepared simulated seawater with fixed a NaSO
4 concentration (28.00 mmol/L) and different NaHCO
3 concentrations (0.00, 2.00, 4.00 mmol/L) to evaluate its ability to quantify HCO
3− in seawater. The corresponding concentrations of HCO
3− are 0.00, 1.93 and 3.79 mmol/L. The corresponding spectra are shown in
Figure 3c, and the detailed spectra are shown in
Figure 3d. Compared with the intensity of SO
42−, the intensity of the HCO
3− signal is much weaker. Even so, the Raman signal of HCO
3− can be detected in the spectrum of the mixed solution with 1.93 mmol/L NaHCO
3. This shows the ability of the NC-CERS system for direct HCO
3− detection in seawater, but the strong and adjacent SO
42− signal of seawater has an impact on the quantitative analysis of HCO
3−.
In order to reduce this impact, we developed an adaptive signal extraction method for HCO
3− and the process is shown in
Figure 4a–c. The spectrum is obtained from a mixed solution with 28.00 mmol/L Na
2SO
4 and 1.93 mmol/L NaHCO
3. In this spectrum, the Raman peaks of SO
42− (981 cm
−1) and H
2O (1640 cm
−1) can be clearly recognized, while the peak of HCO
3− (1021 cm
−1) is almost submerged in the background. Given this, the original spectrum is normalized according to the peak intensity of H
2O to reduce the impact of laser power fluctuation as shown in
Figure 4a. The next step is baseline correction. We have compared four baseline fitting results with four methods (Voigt, Gaussian, Lorentzian and bi-exponential fitting methods), and the polynomial function shows the best performance among them. The baseline corrected result is shown as a blue line in
Figure 4b. The last step is the weak HCO
3− signal extraction from the neighboring strong SO
42− signal. The right wing of the SO
42− signal is regarded as the baseline of the HCO
3− signal and is fitted by using a bi-exponential function in the region of 1000–1050 cm
−1. We again tried four baseline fitting methods (Voigt, Gaussian, Lorentzian and bi-exponential fitting methods), and the polynomial function showed the best performance among them. The fitted baseline is shown as the green dotted line, and the corrected spectrum is shown as the black circle dots in
Figure 4c. The Raman peak of HCO
3− is extracted and fitted by using a Gaussian function which is shown as the red line in
Figure 4c.
Based on this data processing method, the Raman signals of HCO
3− in simulated seawater solutions are extracted and shown in
Figure 4d. The signal of a deep seawater sample is also shown as the cyan line in
Figure 4d. The linear relationship between concentrations and peak intensities of fitted HCO
3− is shown in
Figure 4e and it shows high linearity with R
2 = 0.951 over the range. We chose a mixed solution with 28.00 mmol/L NaSO
4 and 1.50 mmol/L NaHCO
3 as a blind sample. The test result is 1.35 mmol/L with 5.0% relative error, which is shown as the pink point in
Figure 4e. Furthermore, we tested deep seawater, knowing the concentration of HCO
3− is 1.91 mmol/L which is shown as a blue point in
Figure 4e. Compared with the reported HCO
3− concentration value of 1.95 mmol/L in seawater [
4], the relative error was about 2.1%.