Molecularly Imprinted Sol-Gel-Based QCM Sensor Arrays for the Detection and Recognition of Volatile Aldehydes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MISGs
2.3. Coating of Sensing Layer
2.4. QCM Measurement
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdul-Wahab, S.A. Sick Building Syndrome in Public Buildings and Workplaces; Springer: Heidelberg, Germany, 2011. [Google Scholar]
- Ligor, M.; Ligor, T.; Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Denz, H.; Fiegl, M.; Hilbe, W.; Weiss, W.; et al. Determination of Volatile Organic Compounds in Exhaled Breath of Patients With Lung Cancer Using Solid Phase Microextraction and Gas Chromatography Mass Spectrometry. Clin. Chem. Lab. Med. 2009, 47, 550–560. [Google Scholar]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath Gas Aldehydes as Biomarkers of Lung Cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, J.K.; Schelegle, E.S.; Davis, C.E.; Walby, W.F.; Zhao, W.X.; Aksenov, A.A.; Pasamontes, A.; Figueroa, J.; Allen, R. Analysis of Volatile Compounds in Exhaled Breath Condensate in Patients with Severe Pulmonary Arterial Hypertension. PLoS ONE 2014, 9, e95331. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Shi, D.Y.; Ionescu, R.; Zhang, W.; Hua, Q.L.; Pan, Y.Y.; Tao, L.; Liu, H.; Haick, H. Assessment of Ovarian Cancer Conditions from Exhaled Breath. Int. J. Cancer 2015, 136, E614–E622. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Bermudez, E.; Cueto, R.; Squadrito, G.L. Detection of Aldehydes in Bronchoalveolar Lavage of Rats Exposed To Ozone. Fund. Appl. Toxicol. 1996, 34, 148–156. [Google Scholar] [CrossRef]
- Andreoli, R.; Manini, P.; Corradi, M.; Mutti, A.; Niessen, W.M.A. Determination of Patterns of Biologically Relevant Aldehydes in Exhaled Breath Condensate of Healthy Subjects by Liquid Chromatography/Atmospheric Chemical Ionization Tandem Mass Spectrometry. Rapid Commun. Mass. Spetrom. 2003, 17, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Corradi, M.; Rubinstein, I.; Andreoli, R.; Manini, P.; Caglieri, A.; Poli, D.; Alinovi, R.; Mutti, A. Aldehydes in Exhaled Breath Condensate of Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care 2003, 167, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Corradi, M.; Pignatti, P.; Manini, P.; Andreoli, R.; Goldoni, M.; Poppa, M.; Moscato, G.; Balbi, B.; Mutti, A. Comparison Between Exhaled and Sputum Oxidative Stress Biomarkers in Chronic Airway Inflammation. Eur. Respir. J. 2004, 24, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Jareno-Esteban, J.J.; Munoz-Lucas, M.A.; Carrillo-Aranda, B.; Maldonado-Sanz, J.A.; De Granda-Orive, I.; Aguilar-Ros, A.; Civera-Tejuca, C.; Gutierrez-Ortega, C.; Callol-Sanchez, L.M.; Estudio, G. Volatile Organic Compounds in Exhaled Breath in a Healthy Population: Effect of Tobacco Smoking. Arch. Bronconeumol. 2013, 49, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Ohara, K.; Ohshima, T.; Ushio, H. Linear Chain Aldehydes Evoke Calcium Responses in B16 Melanoma Cells. Excli. J. 2011, 10, 303–311. [Google Scholar]
- Itoh, T.; Nakashima, T.; Akamatsu, T.; Izu, N.; Shin, W. Nonanal Gas Sensing Properties of Platinum, Palladium, and Gold-Loaded Tin Oxide VOCs Sensors. Sens. Actuators B Chem. 2013, 187, 135–141. [Google Scholar] [CrossRef]
- Masuda, Y.; Itoh, T.; Shin, W.; Kato, K. SnO2 Nanosheet/Nanoparticle Detector for the Sensing of 1-Nonanal Gas Produced by Lung Cancer. Sci. Rep. 2015, 5, 10122. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, E.L.; Bright, F.V. Molecularly Imprinted Xerogels as Platforms for Sensing. Accounts Chem. Res. 2007, 40, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, E.L.; Bright, F.V. Molecularly Templated Materials in Chemical Sensing. Anal. Chim. Acta 2007, 594, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ge, Y.; Piletsky, S.A.; Lunec, J. Molecularly Imprinted Sensors: Overview and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Matsuguchi, M.; Uno, T. Molecular Imprinting Strategy for Solvent Molecules and Its Application for QCM-Based VOC Vapor Sensing. Sens. Actuators B Chem. 2006, 113, 94–99. [Google Scholar] [CrossRef]
- Bunte, G.; Hurttlen, J.; Pontius, H.; Hartlieb, K.; Krause, H. Gas Phase Detection of Explosives such as 2,4,6-Trinitrotoluene by Molecularly Imprinted Polymers. Anal. Chim. Acta 2007, 591, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Latif, U.; Rohrer, A.; Lieberzeit, P.A.; Dickert, F.L. QCM Gas Phase Detection with Ceramic Materials-VOCs and Oil Vapors. Anal. Bioanal. Chem. 2011, 400, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Iqbal, N.; Mujahid, A.; Schirhagl, R. Advanced Vapor Recognition Materials for Selective and Fast Responsive Surface Acoustic Wave Sensors: A Review. Anal. Chim. Acta 2013, 787, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Hayashi, K. A Quick Responding Quartz Crystal Microbalance Sensor Array Based on Molecular Imprinted Polyacrylic Acids Coating For Selective Identification of Aldehydes in Body Odor. Talanta 2015, 134, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Hayashi, K. Polyacrylic Acid Polymer and Aldehydes Template Molecule Based Mips Coated QCM Sensors for Detection of Pattern Aldehydes in Body Odor. Sens. Actuators B Chem. 2015, 206, 471–487. [Google Scholar] [CrossRef]
- Chen, B.; Liu, C.; Ge, L.; Hayashi, K. Localized Surface Plasmon Resonance Gas Sensor of Au Nano-Islands Coated with Molecularly Imprinted Polymer: Influence of Polymer Thickness on Sensitivity and Selectivity. Sens. Actuators B Chem. 2016, 231, 787–792. [Google Scholar] [CrossRef]
- Chen, B.; Liu, C.; Hayashi, K. Selective Terpene Vapor Detection Using Molecularly Imprinted Polymer Coated Au Nanoparticle LSPR Sensor. IEEE Sens. J. 2014, 14, 3458–3464. [Google Scholar] [CrossRef]
- Chen, B.; Liu, C.; Xie, Y.; Jia, P.; Hayashi, K. Localized Surface Plasmon Resonance Gas Sensor Based on Molecularly Imprinted Polymer Coated Au Nano-Island Films: Influence of Nanostructure on Sensing Characteristics. IEEE Sens. J. 2016, 16, 3532–3540. [Google Scholar]
- Sun, Y. Molecularly imprinted polymer for 2, 4-dichlorophenoxyacetic acid prepared by a sol-gel method. J. Chem. Sci. 2014, 126, 1005–1011. [Google Scholar] [CrossRef]
- Lofgreen, J.E.; Ozin, G.A. Controlling Morphology and Porosity to Improve Performance of Molecularly Imprinted Sol-Gel Silica. Chem. Soc. Rev. 2014, 43, 911–933. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, E.L.; Stratis-Cullum, D.N.; Hankus, M.E. Xerogel-Based Molecularly Imprinted Polymers for Explosives Detection. Proc. SPIE 2010, 7665. [Google Scholar] [CrossRef]
- Mujahid, A.; Lieberzeit, P.A.; Dickert, F.L. Chemical Sensors Based on Molecularly Imprinted Sol-Gel Materials. Materials 2010, 3, 2196–2217. [Google Scholar] [CrossRef]
- Martinez-Manez, R.; Sancenon, F.; Biyikal, M.; Hecht, M.; Rurack, K. Mimicking Tricks from Nature with Sensory Organic-Inorganic Hybrid Materials. J. Mater. Chem. 2011, 21, 12588–12604. [Google Scholar] [CrossRef]
- Díaz-García, E.M.; Laínño, B.R. Molecular Imprinting in Sol-Gel Materials: Recent Developments and Applications. Microchim. Acta. 2004, 149, 19–36. [Google Scholar] [CrossRef]
- Dickert, F.; Greibl, W.; Rohrer, A.; Voigt, G. Sol-Gel-Coated Quartz Crystal Microbalances for Monitoring Automotive Oil Degradation. Adv. Mater. 2001, 13, 1327–1330. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Glanznig, G.; Leidl, A.; Voigt, G.; Dickert, F.L. Nanostructured Polymers for Detecting Chemical Changes During Engine Oil Degradation. IEEE Sens. J. 2006, 6, 529–535. [Google Scholar] [CrossRef]
- Wang, J.; Thongngamdee, S.; Lu, D. Sensitive Voltammetric Sensing of the 2, 3-Dimethyl-2, 3-dinitrobutane (Dmnb) Explosive Taggant. Electroanalysis 2006, 18, 971–975. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Afzal, A.; Glanzing, G.; Dickert, F.L. Molecularly Imprinted Sol-Gel Nanoparticles for Mass-Sensitive Engine Oil Degradation Sensing. Anal. Bioanal. Chem. 2007, 389, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Afzal, A.; Glanzing, G.; Leidl, A.; Lieberzeit, P.A.; Dickert, F.L. Imprinted Sol-Gel Materials for Monitoring Degradation Products in Automotive Oils by Shear Transverse Wave. Anal. Chim. Acta 2010, 675, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, E.L.; Stratis-Cullum, D.N.; Hankus, M.E. A Nanosensor for TNT Detection Based on Molecularly Imprinted Polymers and Surface Enhanced Raman Scattering. Sensors 2011, 11, 2700–2714. [Google Scholar] [CrossRef] [PubMed]
- Latif, U.; Dickert, F.L. Conductometric Sensors for Monitoring Degradation of Automotive Engine Oil. Sensors 2011, 11, 8611–8625. [Google Scholar] [CrossRef] [PubMed]
- Lieberzeit, P.A.; Glanznig, G.; Leidl, A.; Dickert, F.L. Ceramic Materials for Mass-Sensitive Sensors-Detection of VOCs and Monitoring Oil Degradation. Adv. Sci. Technol. 2006, 45, 1799–1802. [Google Scholar] [CrossRef]
- Marteau, C.; Ruyffelaere, F.; Aubry, J.M.; Penverne, C.; Favier, D.; Nardello-Rataj, V. Oxidative Degradation of Fragtant Aldehydes. Autoxidation by Molecular Oxygen. Tetrahedron 2013, 69, 2268–2275. [Google Scholar]
- Jha, S.K.; Liu, C.J.; Hayashi, K. Molecular imprinted polyacrylic acids based QCM sensor array for recognition of organic acids in body odor. Sens. Actuators B Chem. 2014, 204, 74–87. [Google Scholar] [CrossRef]
- Osbourn, G.C.; Martinez, R.F.; Bartholomew, J.W.; Yelton, W.G.; Ricco, A.J. Optimizing chemical sensor array sizes. J. Proc. Electrochem. Soc. 1999, 99, 127–131. [Google Scholar]
- Chaudry, A.N.; Hawkins, T.M.; Travers, P.J. A Method For Selecting an Optimum Sensor Array. Sens. Actuators B Chem. 2000, 69, 236–242. [Google Scholar] [CrossRef]
- Shi, X.J.; Wang, L.Y.; Kariuki, N.; Luo, J.; Zhong, C.J.; Lu, S. A Multi-Module Artificial Neural Network Approach to Pattern Recognition with Optimized Nanostructured Sensor Array. Sens. Actuators B Chem. 2006, 117, 65–73. [Google Scholar] [CrossRef]
- Petersson, H.; Klingvall, R.; Holmberg, M. Sensor Array Optimization Using Variable Selection and a Scanning Light Pulse Technique. Sens. Actuators B Chem. 2009, 142, 435–445. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, C.; Zeng, D.; Li, H.; Liu, Y.; Cai, S. A Sensor Array Optimization Method for Electronic Noses with Sub-arrays. Sens. Actuators B Chem. 2009, 142, 243–252. [Google Scholar] [CrossRef]
- Cen, L.; Ser, W.; Yu, Z.L.; Rahardja, S.; Cen, W. Linear Sparse Array Synthesis with Minimum Number of Sensors. IEEE Trans. Antenn. Propag. 2010, 58, 720–726. [Google Scholar]
- Muezzinoglu, M.K.; Vergara, A.; Huerta, R.; Rabinovich, M.I. A Sensor Conditioning Principle for Odor Identification. Sens. Actuators B Chem. 2010, 146, 472–476. [Google Scholar] [CrossRef]
- Hossain, M.E.; Rahman, G.M.A.; Freund, M.S.; Jayas, D.S.; White, N.D.G.; Shafai, C.; Thomson, D.J. Fabrication and Optimization of a Conducting Polymer Sensor Array Using Stored Grain Model Volatiles. J. Agric. Food Chem. 2012, 60, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.L.; Zhao, L.; Zhi, R.C.; Xi, X.J. Optimization of Electronic Nose Sensor Array by Genetic Algorithms in Xihu-Longjing Tea Quality Analysis. Math. Comput. Model 2013, 58, 746–752. [Google Scholar] [CrossRef]
- Johnson, K.J.; Rose-Pehrsson, S.L. Sensor Array Design for Complex Sensing Tasks. Annu. Rev. Anal. Chem. 2015, 8, 287–310. [Google Scholar] [CrossRef] [PubMed]
- Pearce, T.C.; Schiffman, S.S.; Nagle, H.T.; Gardner, J.W. Handbook of Machine Olfaction: Electronic Nose Technology; John Wiley & Sons: San Francisco, CA, USA, 2006. [Google Scholar]

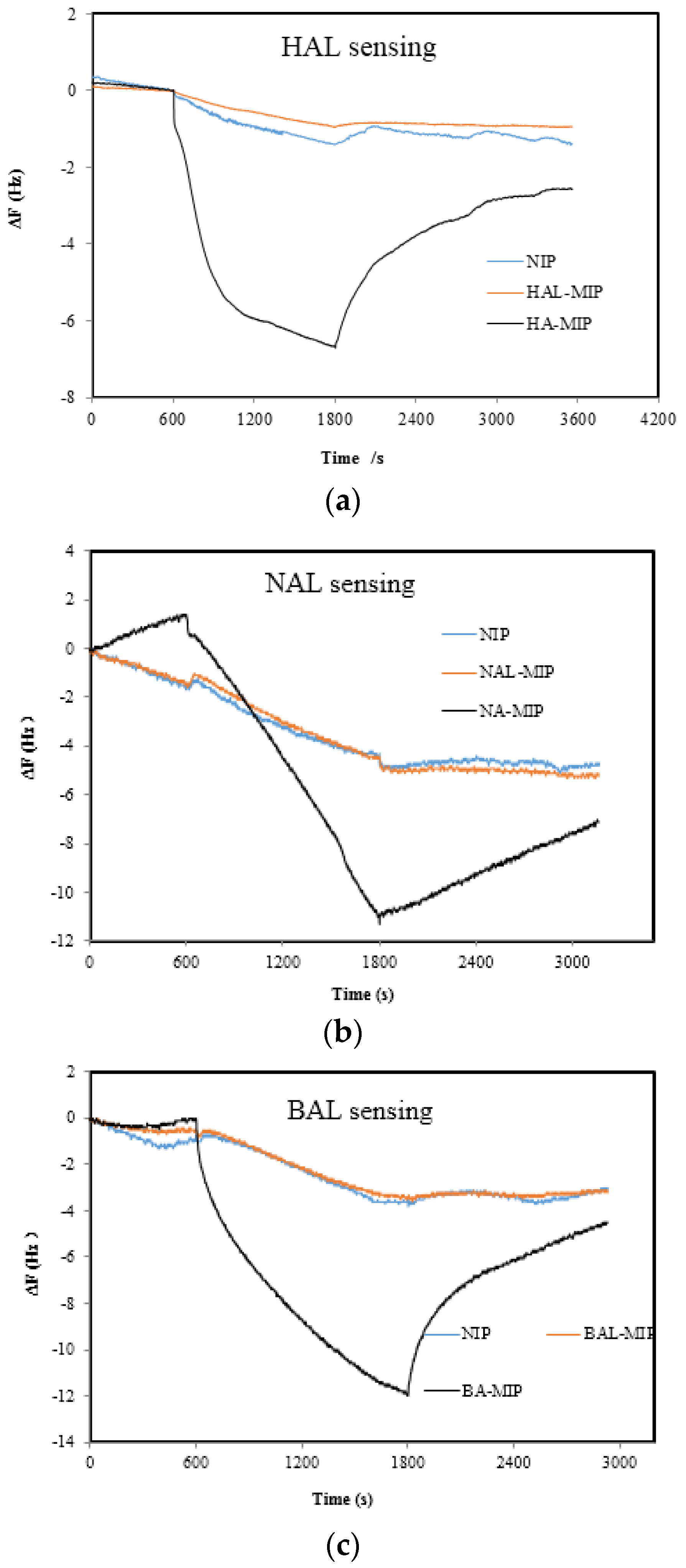
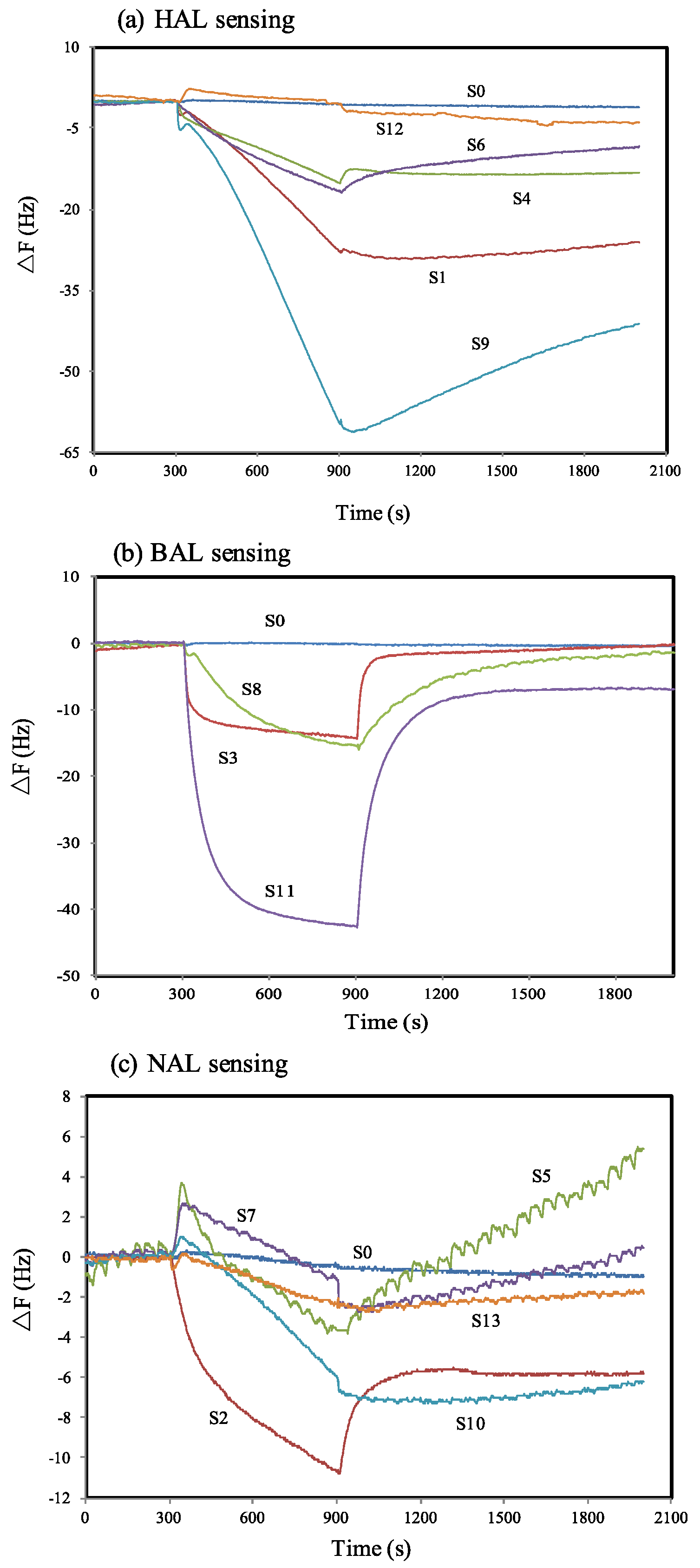

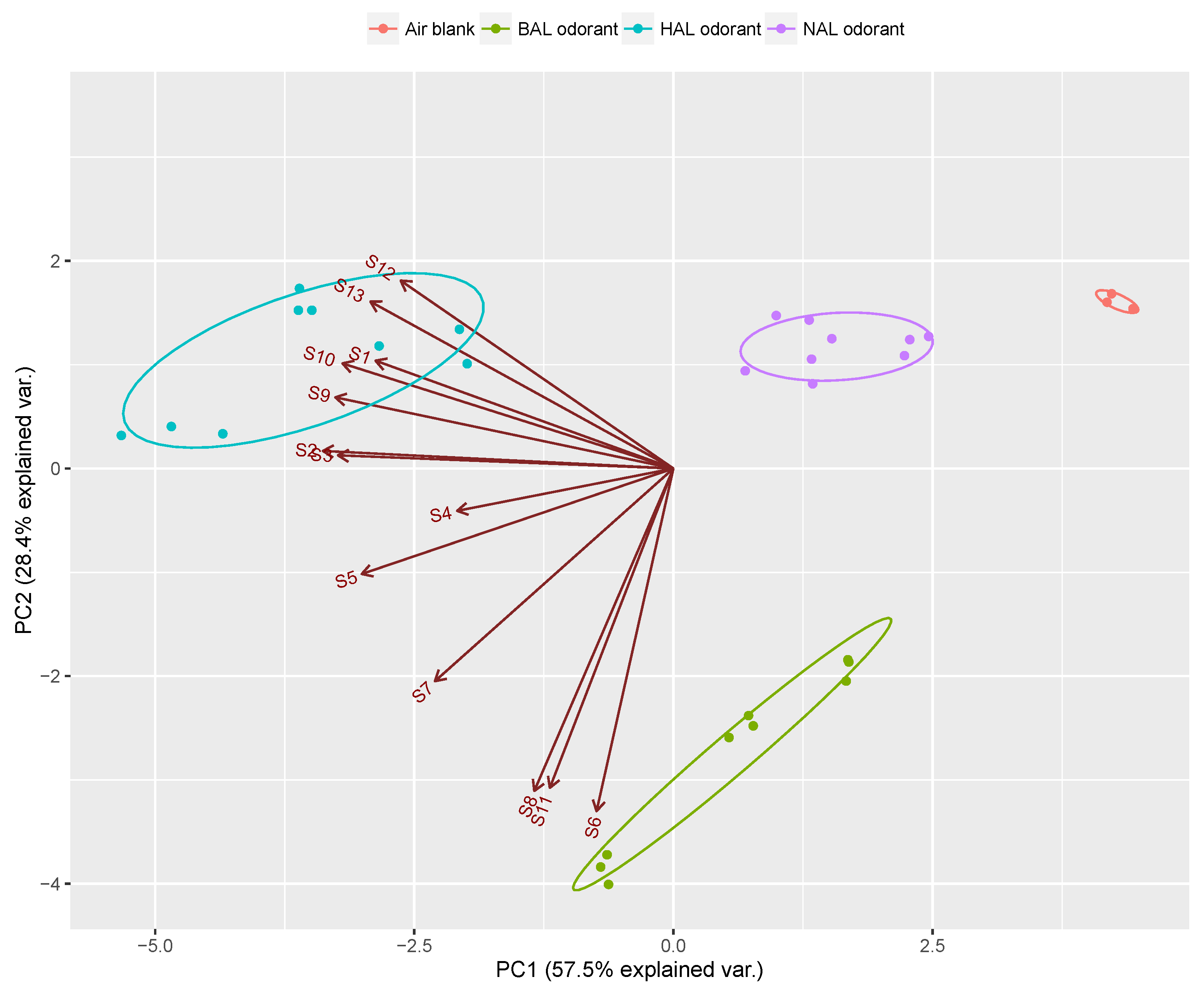


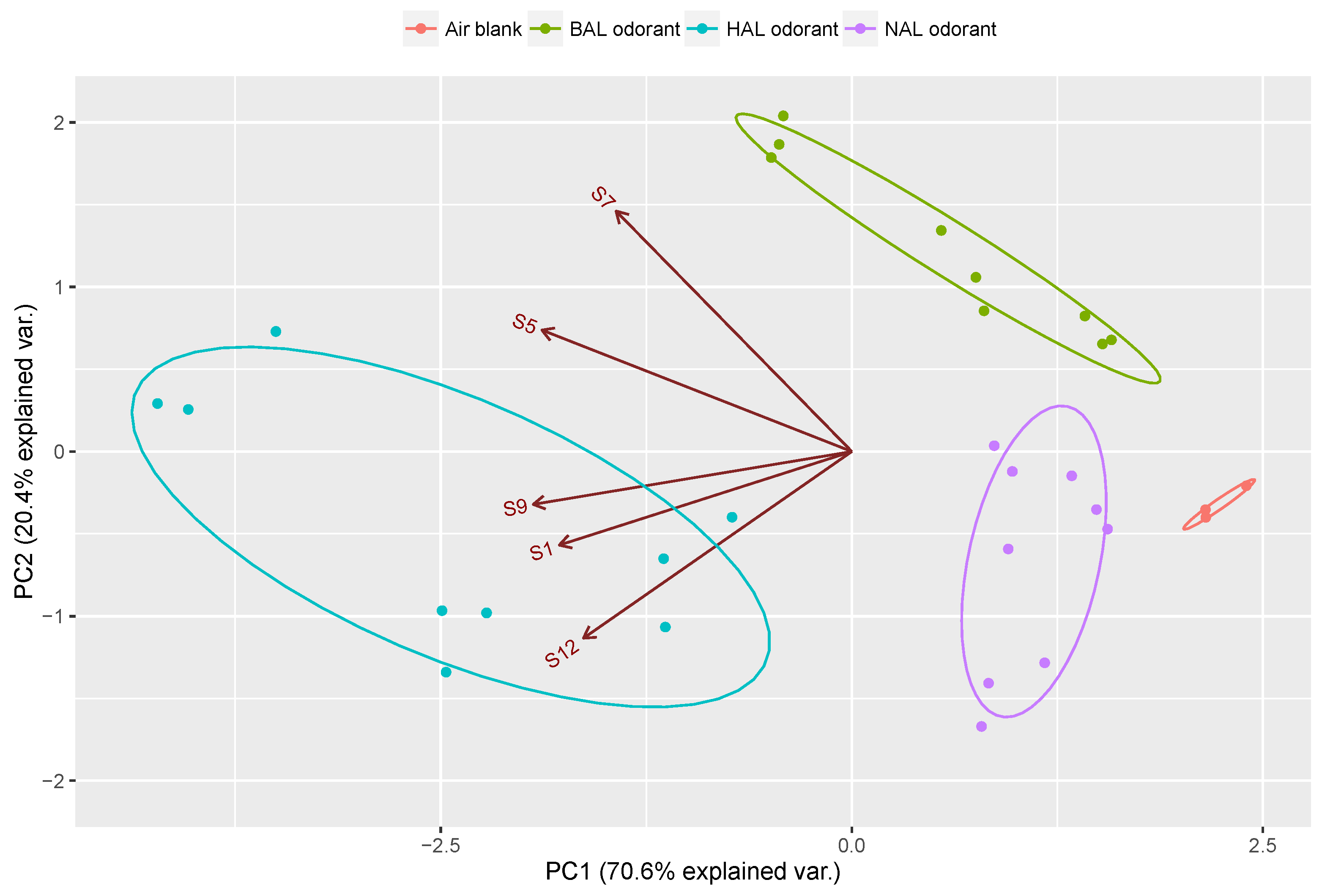
| Sensor Number | Sensor Name Abbreviation | Matrix Materials | Functional Monomers | Template Molecules |
|---|---|---|---|---|
| S0 | Ti-Blank (NIP) | TBOT | - | - |
| S1 | Ti-HA-MIP | TBOT | - | HA |
| S2 | Ti-NA-MIP | TBOT | - | NA |
| S3 | Ti-BA-MIP | TBOT | - | BA |
| S4 | Ti-APT-HA-MIP | TBOT | APT | HA |
| S5 | Ti-APT-NA-MIP | TBOT | APT | NA |
| S6 | Ti-EPA-HA-MIP | TBOT | EPA | HA |
| S7 | Ti-EPA-NA-MIP | TBOT | EPA | NA |
| S8 | Ti-TMP-BA-MIP | TBOT | TMP | BA |
| S9 | Si-EPA-HA-MIP | TEOS | EPA | HA |
| S10 | Si-EPA-NA-MIP | TEOS | EPA | NA |
| S11 | Si-TMP-BA-MIP | TEOS | TMP | BA |
| S12 | Si-APT-HA-MIP | TEOS | APT | HA |
| S13 | Si-APT-NA-MIP | TEOS | APT | NA |
| Sensor | F-Value | Pr | MISG |
|---|---|---|---|
| S10 | 350.50 | 2.20 × 10−16 | Si-EPA-NA-MIP |
| S9 | 283.69 | 2.20 × 10−16 | Si-EPA-HA-MIP |
| S6 | 104.36 | 1.28 × 10−14 | Ti-EPA-HA-MIP |
| S3 | 87.04 | 1.11 × 10−13 | Ti-BA-MIP |
| S8 | 63.41 | 4.34 × 10−12 | Ti-TMP-BA-MIP |
| S2 | 62.78 | 4.86 × 10−12 | Ti-NA-MIP |
| S13 | 61.69 | 5.92 × 10−12 | Si-APT-NA-MIP |
| S11 | 42.96 | 3.23 × 10−10 | Si-TMP-BA-MIP |
| S12 | 31.07 | 9.43 × 10−9 | Si-APT-HA-MIP |
| S1 | 24.29 | 1.04 × 10−7 | Ti-HA-MIP |
| S4 | 22.80 | 1.89 × 10−7 | Ti-APT-HA-MIP |
| S5 | 15.79 | 4.76 × 10−6 | Ti-APT-NA-MIP |
| S7 | 11.57 | 5.29 × 10−5 | Ti-EPA-NA-MIP |
| Sensor Number | SRSS | SED | |
|---|---|---|---|
| Optimized array | 3,6,8,10,13 | 3.35 | 14.87 |
| Random array | 1,5,7,9,12 | 3.36 | 10.77 |
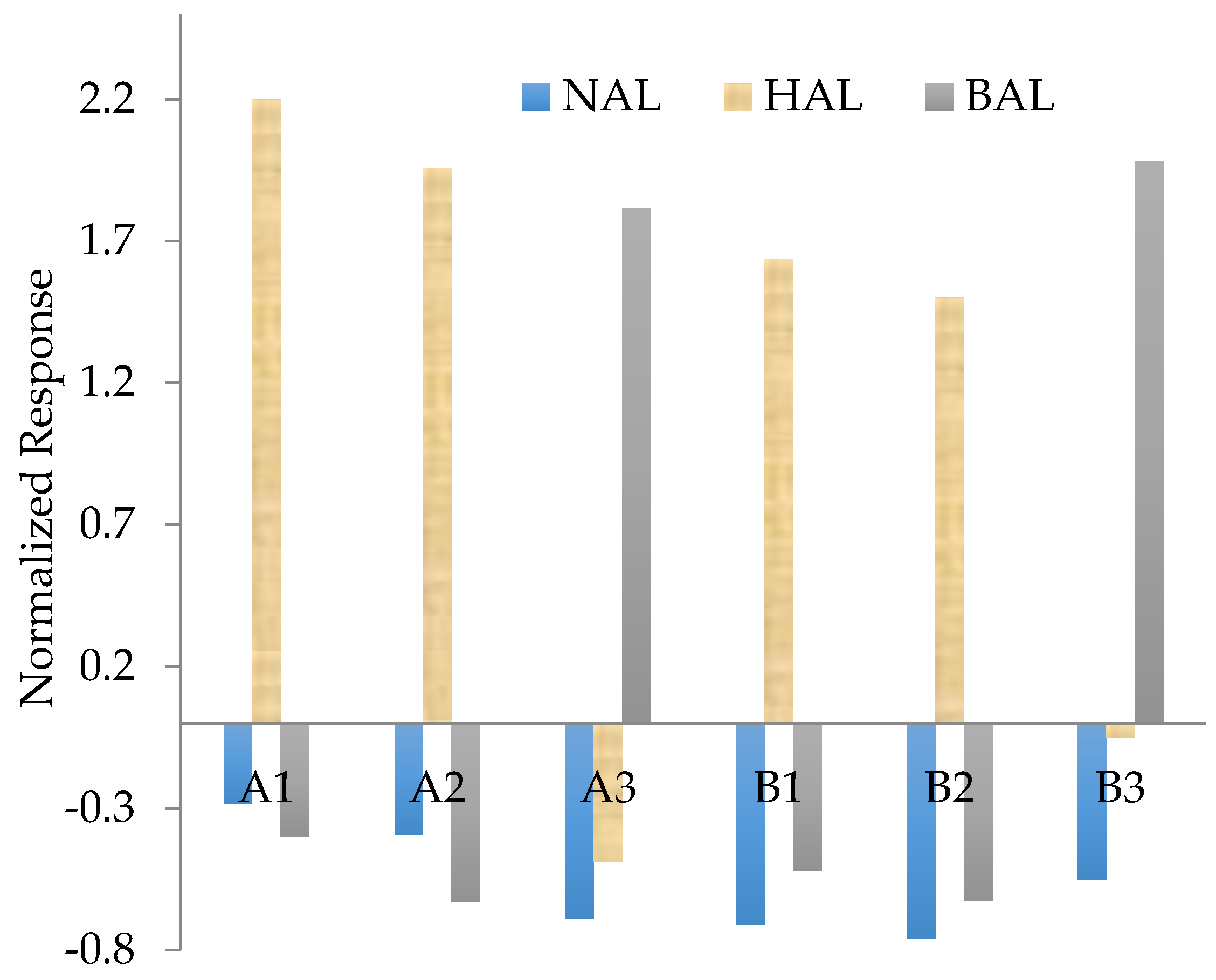
| Sensor Name | Matrix Materials | Functional Monomers | Template Molecules |
|---|---|---|---|
| A1 | TEOS 100 | EPA 100 | HA50 |
| A2 | TEOS 100 | EPA 100 | NA50 |
| A3 | TEOS100 | TMP 100 | BA50 |
| B1 | TEOS 150 | EPA 50 | HA50 |
| B2 | TEOS 150 | EPA 50 | NA50 |
| B3 | TEOS 150 | TMP 50 | BA50 |
| Sensor Array | SRSS | SED |
|---|---|---|
| (A) A1, A2, A3 | 2.34 | 5.07 |
| (B) B1, B2, B3 | 2.15 | 4.77 |
| (C) B1, A2, A3 | 2.32 | 5.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wyszynski, B.; Yatabe, R.; Hayashi, K.; Toko, K. Molecularly Imprinted Sol-Gel-Based QCM Sensor Arrays for the Detection and Recognition of Volatile Aldehydes. Sensors 2017, 17, 382. https://doi.org/10.3390/s17020382
Liu C, Wyszynski B, Yatabe R, Hayashi K, Toko K. Molecularly Imprinted Sol-Gel-Based QCM Sensor Arrays for the Detection and Recognition of Volatile Aldehydes. Sensors. 2017; 17(2):382. https://doi.org/10.3390/s17020382
Chicago/Turabian StyleLiu, Chuanjun, Bartosz Wyszynski, Rui Yatabe, Kenshi Hayashi, and Kiyoshi Toko. 2017. "Molecularly Imprinted Sol-Gel-Based QCM Sensor Arrays for the Detection and Recognition of Volatile Aldehydes" Sensors 17, no. 2: 382. https://doi.org/10.3390/s17020382
APA StyleLiu, C., Wyszynski, B., Yatabe, R., Hayashi, K., & Toko, K. (2017). Molecularly Imprinted Sol-Gel-Based QCM Sensor Arrays for the Detection and Recognition of Volatile Aldehydes. Sensors, 17(2), 382. https://doi.org/10.3390/s17020382






