SVM-Based Spectral Analysis for Heart Rate from Multi-Channel WPPG Sensor Signals
Abstract
:1. Introduction
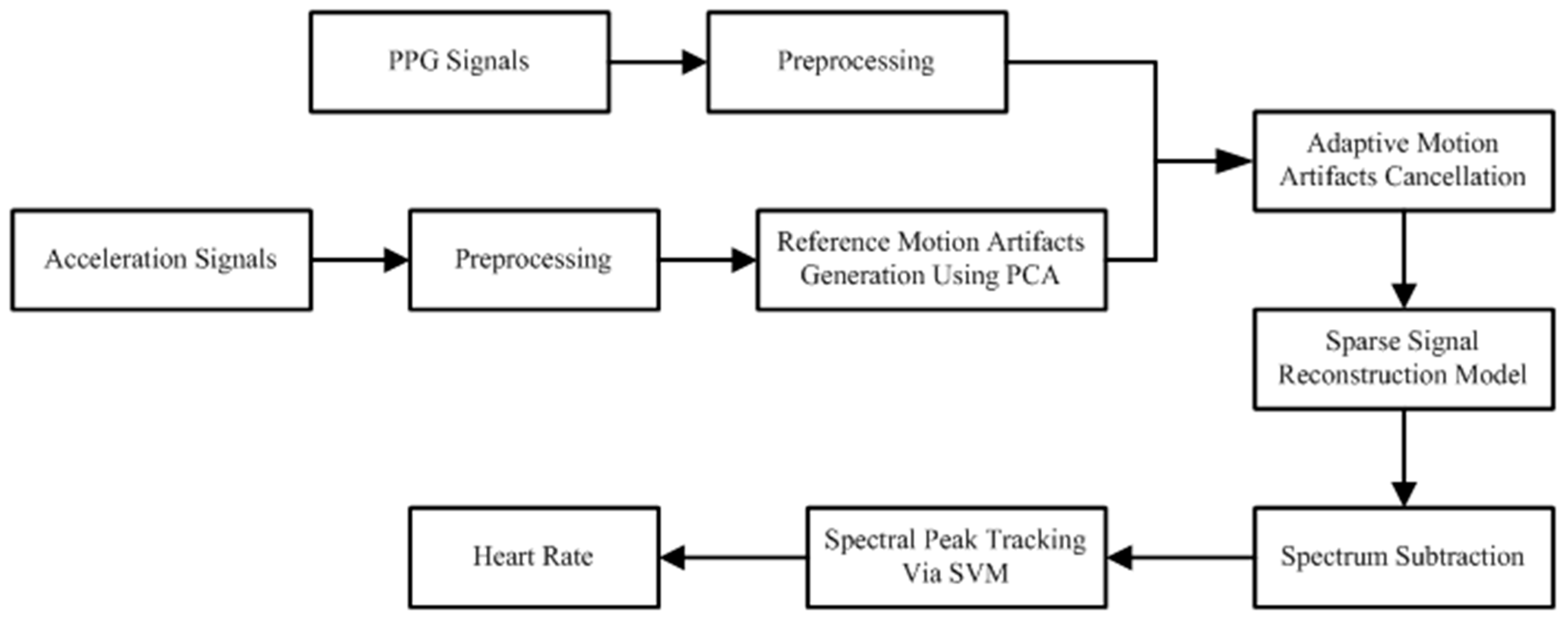
2. Materials and Methods
2.1. Data Description
2.2. Methodology
2.2.1. Preprocessing
2.2.2. Initial Motion Artifacts Reduction
2.2.3. Sparse Signal Reconstruction Model
2.2.4. Spectrum Subtraction
- Step 1:
- We need to seek the maximum value of spectral coefficients in acceleration spectra for each frequency bin .
- Step 2:
- At each frequency bin , the value of the spectral coefficients are subtracted in two WPPG spectra. Within , the maximum values of all coefficients are denoted , in two WPPG spectra, respectively.
- Step 3:
- Within , we set to zero all spectral coefficients with values less than in WPPG1 spectra, and all spectral coefficients with values less than are also set to zero in WPPG2 spectra. Finally, we can yield two cleansed WPPG spectra.
2.2.5. SVM-Based Spectral Analysis
- (1)
- About 75% of spectral peaks that have the largest coefficient in their corresponding time windows are true spectral peaks.
- (2)
- About 84% of spectral peaks that have the shortest distance from their previous true spectral peak are true spectral peaks.
- (3)
- About 96% of true spectral peaks have the largest coefficient and shortest distance.
- (1)
- If only one spectral peak is true, and then we select the spectral peak corresponding to frequency, denoted as .
- (2)
- If the classifier output more than one true spectral peak, we select the spectral peak of the closest to , its frequency is denoted as .
- (3)
- If there is no true spectral peak, we consider that SVM classifier cannot seek out reliable spectral peaks because of serious motion artifacts in the current time window. Hence, a prediction mechanism is proposed for solving the problem. The mechanism can be expressed as follows
3. Results
3.1. Parameter Settings
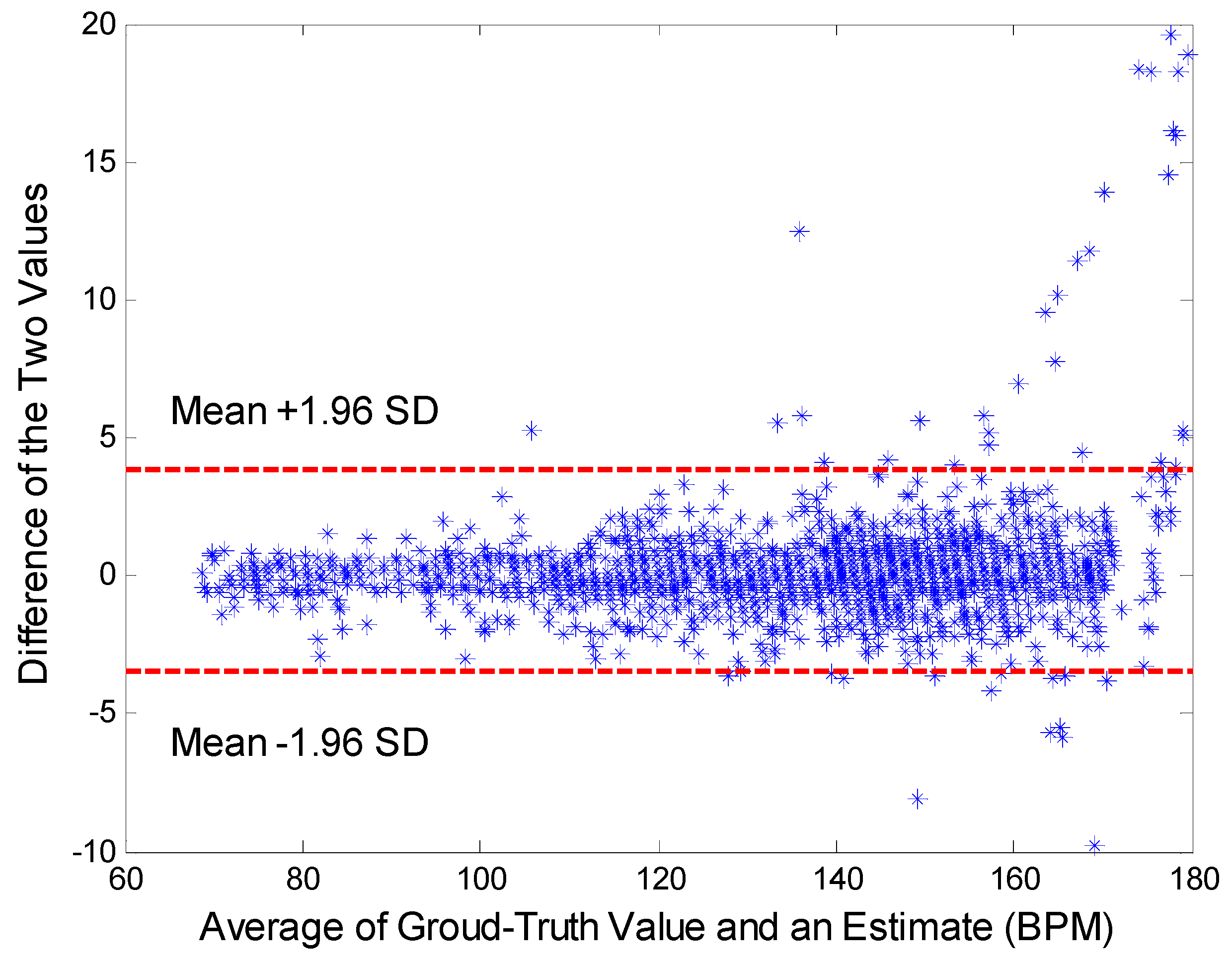
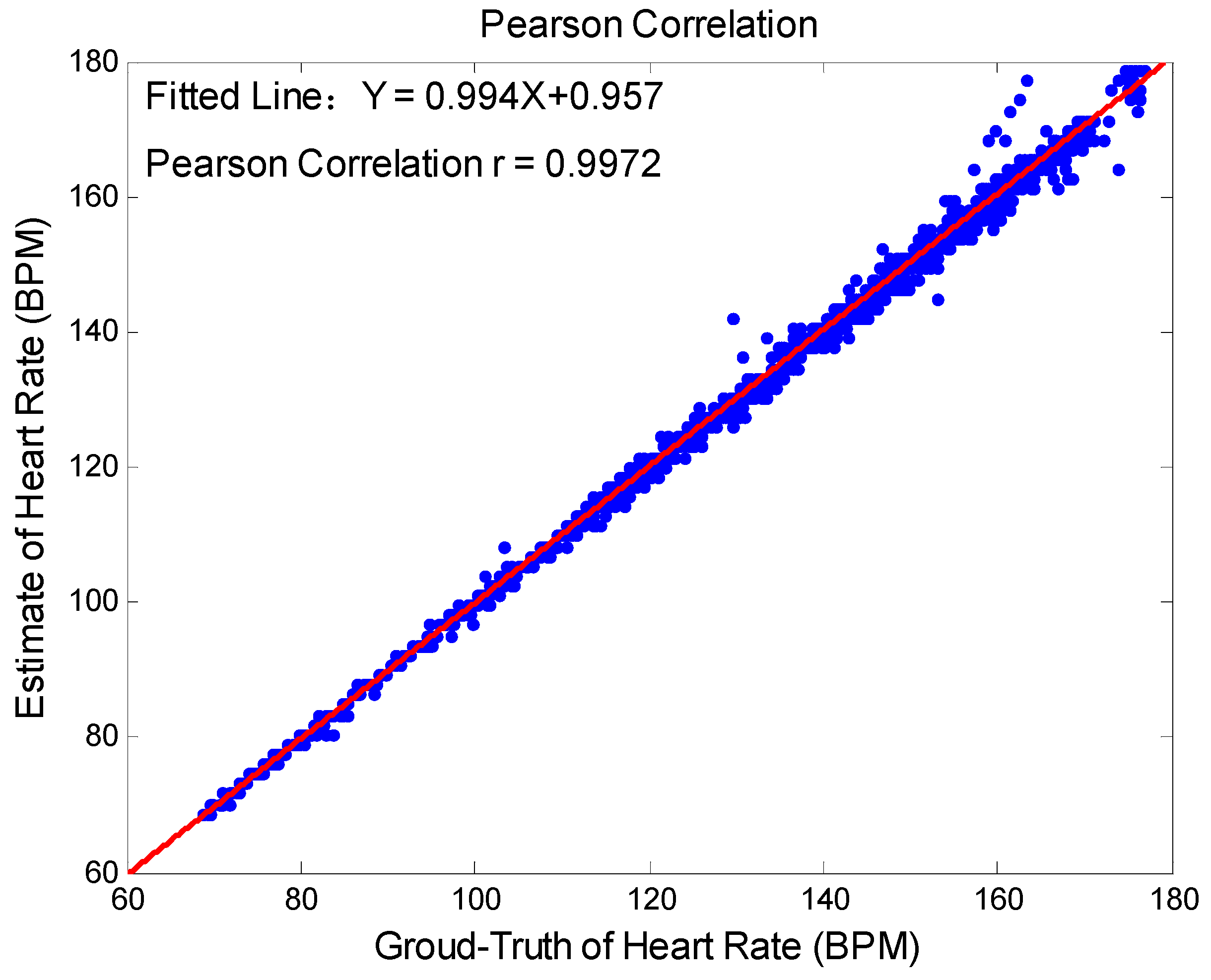
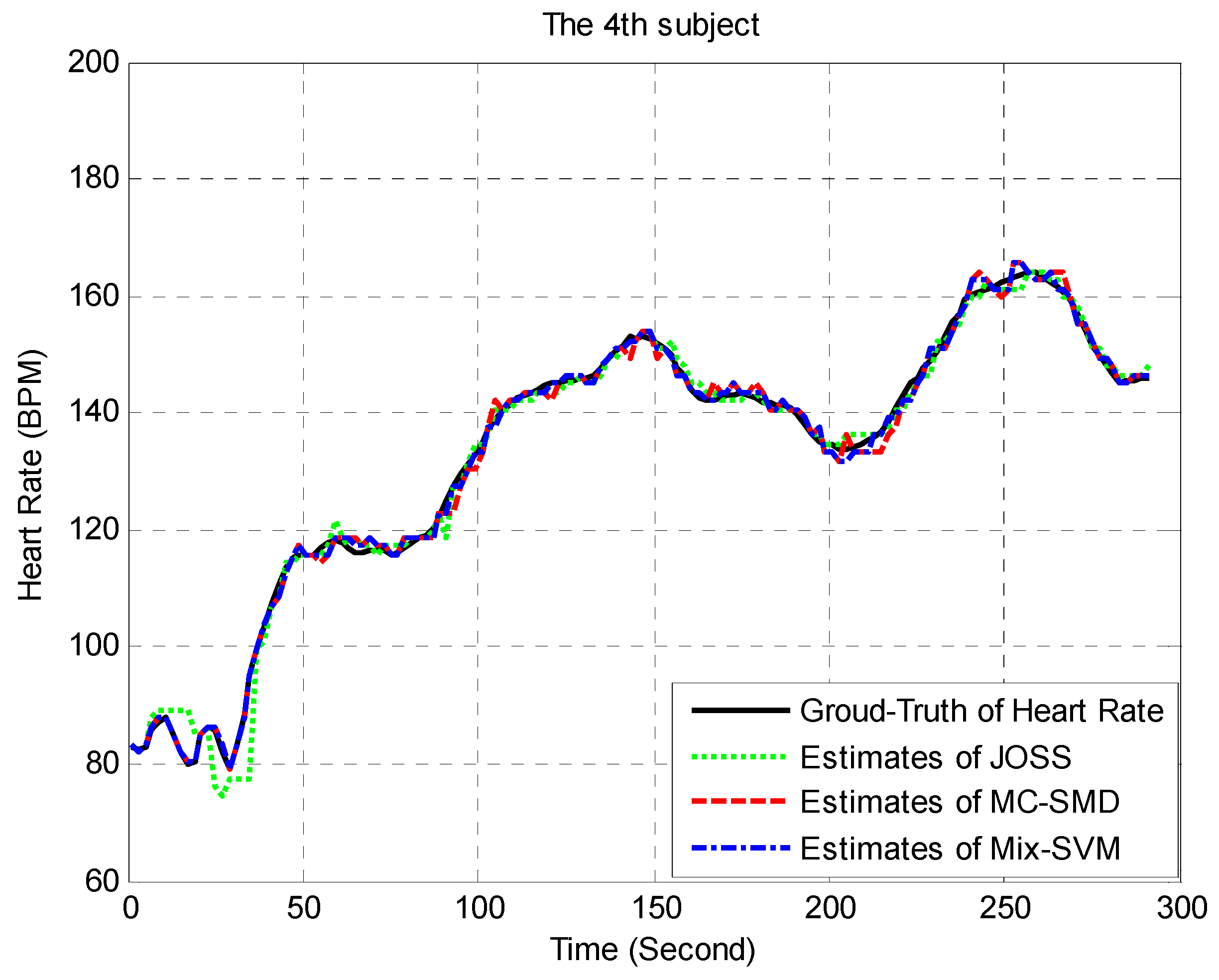
3.2. Data Analysis and Statistics
3.3. Results Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamal, A.A.R.; Harness, J.B.; Irving, G.; Mearns, A.J. Skin photoplethysmography—A review. Comput. Methods Programs Biomed. 1989, 28, 257–269. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.Y.A. Comparison of wavelet transformation and adaptive filtering in restoring artefact-induced time-related measurement. Biomed. Signal Process. Control 2006, 1, 93–98. [Google Scholar] [CrossRef]
- Sahni, R.; Gupta, A.; Ohira-Kist, K. Motion resistant pulse oximetry in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F505–F508. [Google Scholar] [CrossRef] [PubMed]
- Kim, I. Challenges in wearable personal health monitoring systems. Eng. Med. Biol. Soc. 2014, 2014, 5264–5267. [Google Scholar]
- Graybeal, J.M.; Petterson, M.T. Adaptive filtering and alternative calculations revolutionizes pulse oximetry sensitivity and specificity during motion and low perfusion. Eng. Med. Biol. Soc. 2004, 2, 5363–5366. [Google Scholar]
- Poh, M.Z.; Swenson, N.C.; Picard, R.W. Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, R.; Nourani, M.; Ostadabbas, S. A motion-tolerant adaptive algorithm for wearable photoplethysmographic biosensors. IEEE J. Biomed. Health Inf. 2014, 18, 670–681. [Google Scholar] [CrossRef]
- Reddy, K.A.; Kumar, V.J. Motion Artifact Reduction in Photoplethysmographic Signals using Singular Value Decomposition. In Proceedings of the 2007 IEEE Instrumentation and Measurement Technology Conference, Warsaw, Poland, 1–3 May 2007.
- Couceiro, R.; Carvalho, P.; Paiva, R.P. Detection of motion artifact patterns in photoplethysmographic signals based on time and period domain analysis. Physiol. Meas. 2014, 35, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Zhang, Y.T. Reduction of motion artifacts from photoplethysmographic recordings using a wavelet denoising approach. In Proceedings of the IEEE EMBS Asian-Pacific Conference on Biomedical Engineering, Kyoto, Japan, 20–22 October 2003; pp. 194–195.
- Raghuram, M.; Madhav, K.V.; Krishna, E.H. Evaluation of wavelets for reduction of motion artifacts in photoplethysmographic signals. In Proceedings of the 2010 10th International Conference on Information Sciences Signal Processing and their Applications (ISSPA), Kuala Lumpur, Malaysia, 10–13 May 2010; pp. 460–463. [CrossRef]
- Kim, B.S.; Yoo, S.K. Motion artifact reduction in photoplethysmography using independent component analysis. IEEE Trans. Biomed. Eng. 2006, 53, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Zhang, Z. Combining ensemble empirical mode decomposition with spectrum subtraction technique for heart rate monitoring using wrist-type photoplethysmography. Biomed. Signal Process. Control 2015, 21, 119–125. [Google Scholar] [CrossRef]
- Sun, X.; Yang, P.; Li, Y. Robust heart beat detection from photoplethysmography interlaced with motion artifactss based on empirical mode decomposition. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Hong Kong, China, 5–7 January 2012; pp. 775–778.
- Fukushima, H.; Kawanaka, H.; Bhuiyan, M.S. Estimating heart rate using wrist-type photoplethysmography and acceleration sensor while running. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), San Diego, CA, USA, 28 August–1 September 2012; pp. 2901–2904.
- Seyedtabaii, S.; Seyedtabaii, L. Kalman filter based adaptive reduction of motion artifact from photoplethysmographic signal. World Acad. Sci. Eng. Technol. 2008, 37, 173–176. [Google Scholar]
- Yan, Y.S.; Poon, C.C.; Zhang, Y.T. Reduction of motion artifact in pulse oximetry by smoothed pseudo Wigner-Ville distribution. J. Neuroeng. Rehab. 2005, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Silva, S.M.; Giannetti, R.; Dotor, M.L. Heuristic algorithm for photoplethysmographic heart rate tracking during maximal exercise test. J. Med. Biol. Eng. 2012, 32, 181–188. [Google Scholar] [CrossRef]
- Lai, P.H.; Kim, I. Lightweight wrist photoplethysmography for heavy exercise: Motion robust heart rate monitoring algorithm. Healthc. Technol. Lett. 2015, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Heart rate monitoring from wrist-type photoplethysmographic (PPG) signals during intensive physical exercise. In Proceedings of the 2014 IEEE Global Conference on Signal and Information Processing (GlobalSIP), Atlanta, GA, USA, 3–5 December 2014; pp. 698–702.
- Zhang, Z.; Pi, Z.; Liu, B. TROIKA: A general framework for heart rate monitoring using wrist-type photoplethysmographic signals during intensive physical exercise. IEEE Trans. Biomed. Eng. 2014, 62, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Photoplethysmography-based heart rate monitoring in physical activities via joint sparse spectrum reconstruction. IEEE Trans. Biomed. Eng. 2015, 62, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Cai, L.; Jiang, D. Spectral Matrix Decomposition-Base Motion Artifacts Removal in Multi-Channel PPG Sensor Signals. IEEE Access 2016, 4, 3076–3086. [Google Scholar] [CrossRef]
- Tautan, A.M.; Young, A.; Wentink, E.; Wieringa, F. Characterization and reduction of motion artifacts in photoplethysmographic signals from a wrist-worn device. In Proceedings of the 2015 37th IEEE Annual International Conference Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 6146–6149.
- Zhang, Z. Undergraduate student compete in the IEEE signal process cup: Part 3. IEEE Signal Process. Mag. 2015, 32, 113–116. [Google Scholar] [CrossRef]
- Chan, K.W.; Zhang, Y.T. Adaptive reduction of motion artifact from photoplethysmographic recordings using a variable step-size LMS filter. IEEE Sens. 2002, 2, 1343–1346. [Google Scholar]
- Han, H.; Kim, J. Artifacts in wearable photoplethysmographs during daily life motions and their reduction with least mean square based active noise cancellation method. Comput. Biol. Med. 2011, 83, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yao, D. Removal of the ocular artifacts from EEG data using a cascaded spatio-temporal processing. Comput. Methods Programs Biomed. 2006, 83, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Cotter, S.F.; Rao, B.D.; Engan, K. Sparse solutions to linear inverse problems with multiple measurement vectors. IEEE Trans. Signal Process. 2005, 53, 2477–2488. [Google Scholar] [CrossRef]
- Zhang, Z.; Rao, B.D. Sparse Signal Recovery with Temporally Correlated Source Vectors Using Sparse Bayesian Learning. IEEE J. Sel. Top. Signal Process. 2011, 5, 912–926. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Gorodnitsky, I.F.; Rao, B.D. Sparse signal reconstruction from limited data using FOCUSS: A re-weighted minimum norm algorithm. Signal Process. IEEE Trans. 1997, 45, 600–616. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Z. Photoplethysmography-Based Heart Rate Monitoring Using Asymmetric Least Squares Spectrum Subtraction and Bayesian Decision Theory. IEEE. Sens. J. 2015, 15, 7161–7168. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Eilers, P.H.C. A perfect smoother. Anal. Chem. 2003, 75, 3631–3636. [Google Scholar] [CrossRef] [PubMed]

| Methods | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Literature [22] | 2.87 | 2.75 | 1.91 | 2.25 | 1.69 | 3.16 | 1.72 | 1.83 | 1.58 | 4.00 | 1.96 | 3.33 | 2.42 |
| Literature [23] | 1.33 | 1.75 | 1.47 | 1.48 | 0.69 | 1.32 | 0.71 | 0.56 | 0.49 | 3.81 | 0.78 | 1.04 | 1.28 |
| Literature [24] | 1.16 | 1.07 | 0.80 | 1.13 | 0.98 | 1.29 | 0.88 | 0.81 | 0.55 | 3.18 | 0.79 | 0.72 | 1.11 |
| Mix-SVM | 1.08 | 1.00 | 0.69 | 0.86 | 0.80 | 1.24 | 0.90 | 0.52 | 0.48 | 2.95 | 0.80 | 0.75 | 1.01 |
| Methods | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Literature [22] | 2.18 | 2.37 | 1.50 | 2.00 | 1.22 | 2.51 | 1.27 | 1.47 | 1.28 | 2.49 | 1.29 | 2.30 | 1.82 |
| Literature [23] | 1.19 | 1.66 | 1.27 | 1.41 | 0.51 | 1.09 | 0.54 | 0.47 | 0.41 | 2.43 | 0.51 | 0.81 | 1.01 |
| Literature [24] | 0.91 | 0.87 | 0.62 | 0.84 | 0.68 | 0.96 | 0.65 | 0.64 | 0.43 | 1.95 | 0.51 | 0.53 | 0.80 |
| Mix-SVM | 0.86 | 0.81 | 0.53 | 0.65 | 0.55 | 0.92 | 0.66 | 0.42 | 0.40 | 1.79 | 0.52 | 0.55 | 0.72 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J.; Cai, L.; Wang, F.; He, X. SVM-Based Spectral Analysis for Heart Rate from Multi-Channel WPPG Sensor Signals. Sensors 2017, 17, 506. https://doi.org/10.3390/s17030506
Xiong J, Cai L, Wang F, He X. SVM-Based Spectral Analysis for Heart Rate from Multi-Channel WPPG Sensor Signals. Sensors. 2017; 17(3):506. https://doi.org/10.3390/s17030506
Chicago/Turabian StyleXiong, Jiping, Lisang Cai, Fei Wang, and Xiaowei He. 2017. "SVM-Based Spectral Analysis for Heart Rate from Multi-Channel WPPG Sensor Signals" Sensors 17, no. 3: 506. https://doi.org/10.3390/s17030506





