ZnO Film Bulk Acoustic Resonator for the Kinetics Study of Human Blood Coagulation
Abstract
:1. Introduction
2. Device Configuration and Fabrication
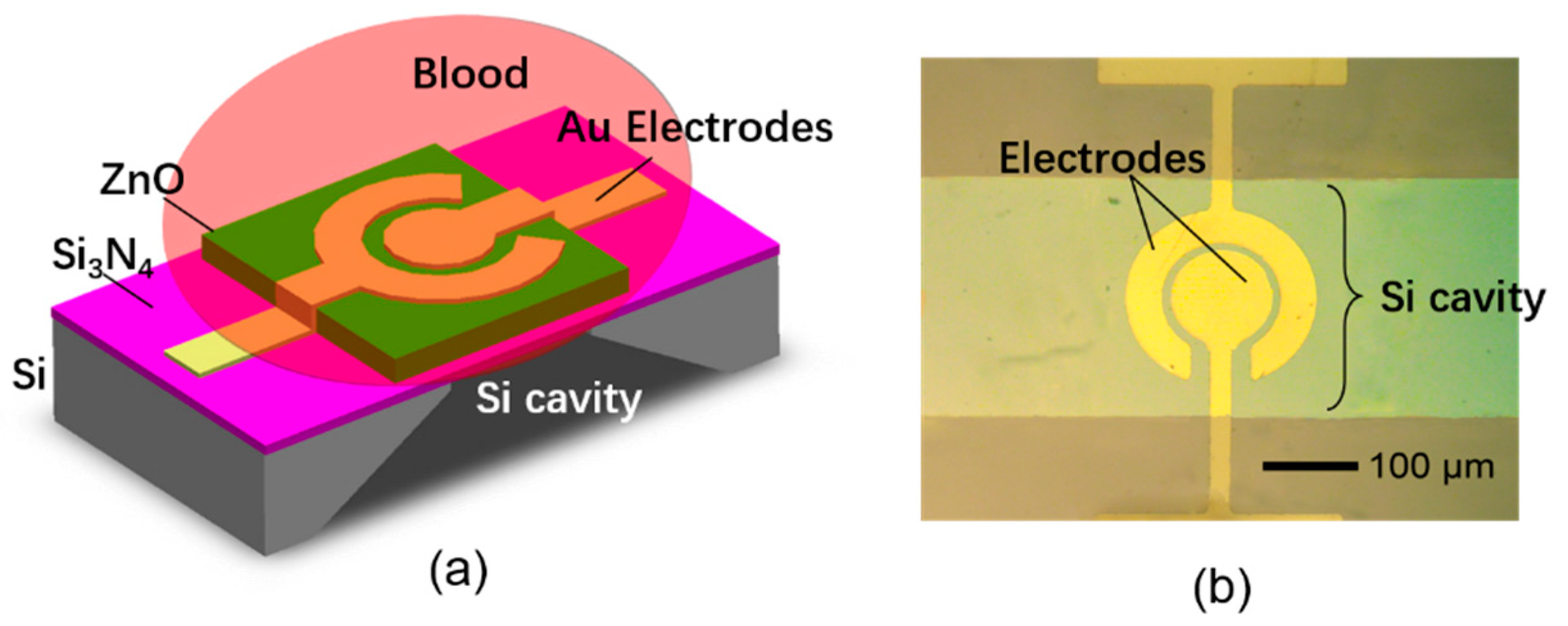
2.1. The Structure of the FBAR Sensor
2.2. The Device Fabrication Process
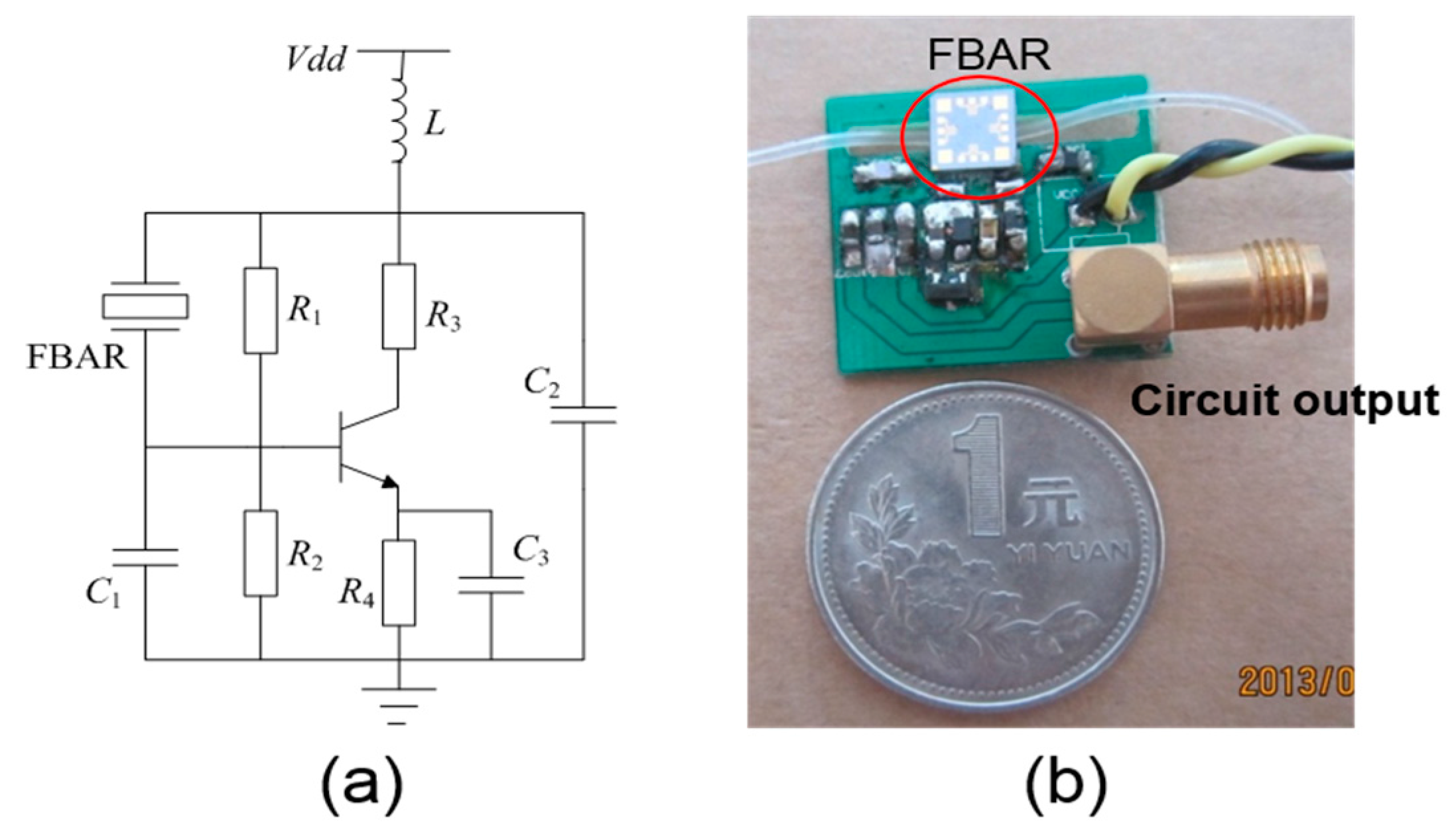
2.3. The Circuit Diagram and Measurement Procedure
3. Results and Discussion
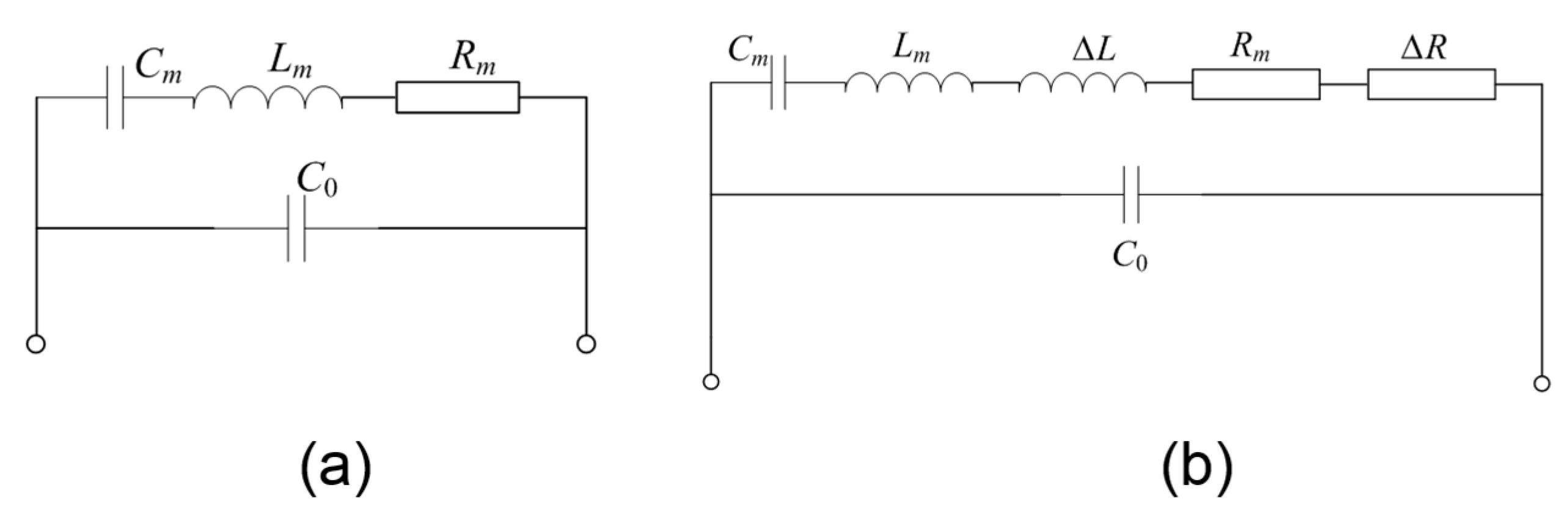
3.1. The Resonant Characterization of the FBAR Device
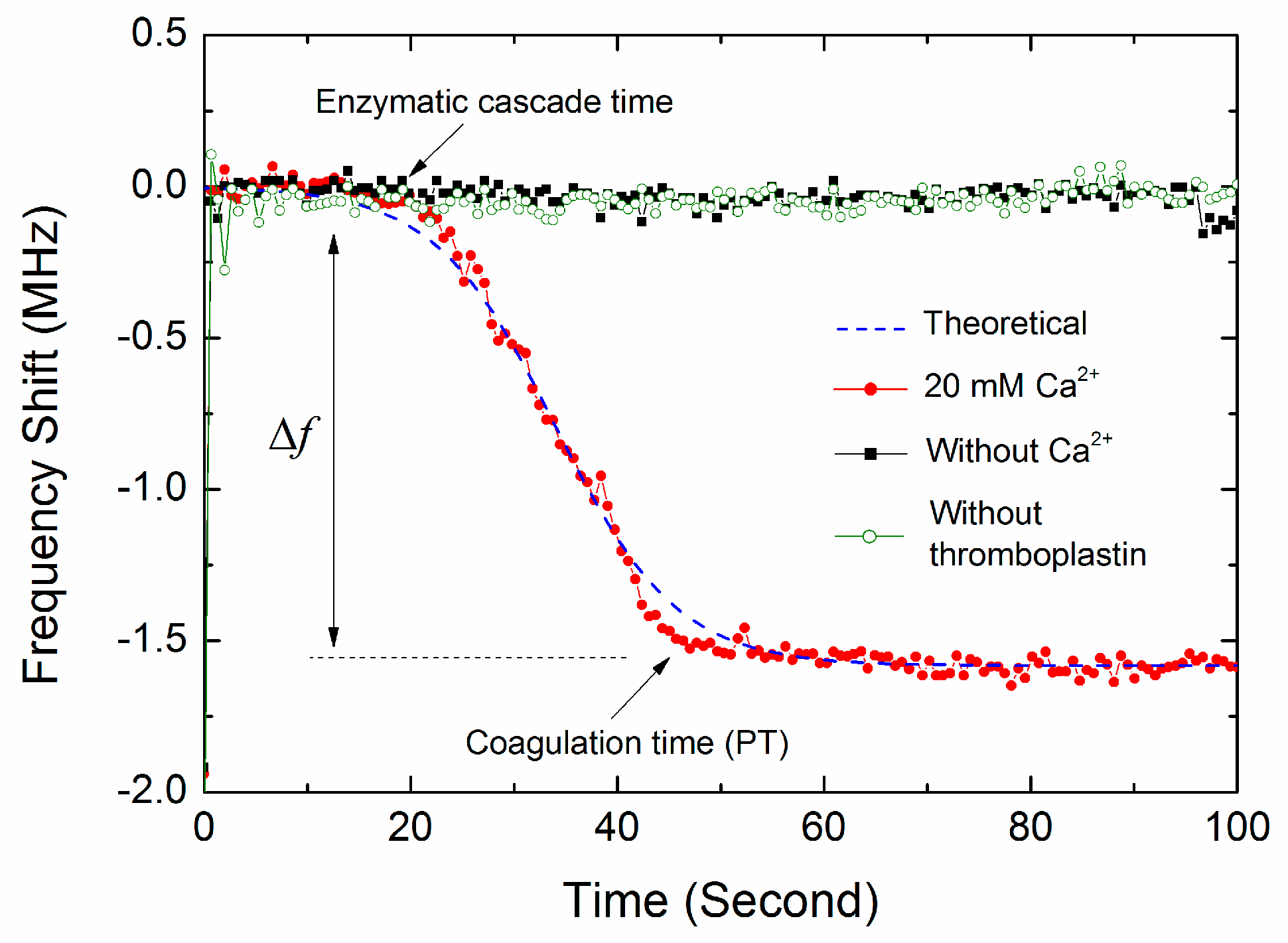
3.2. The Monitoring of Blood Coagulation
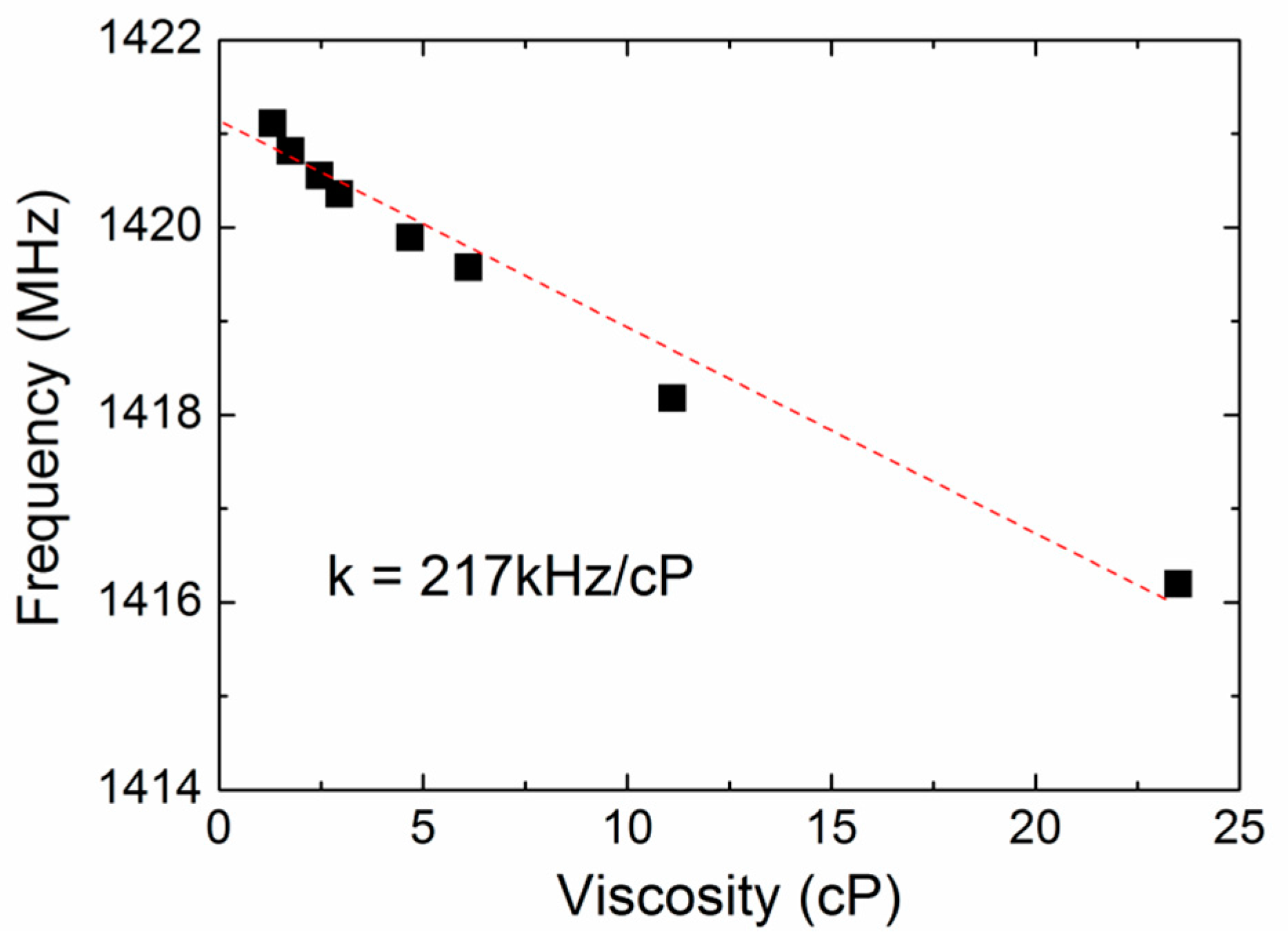
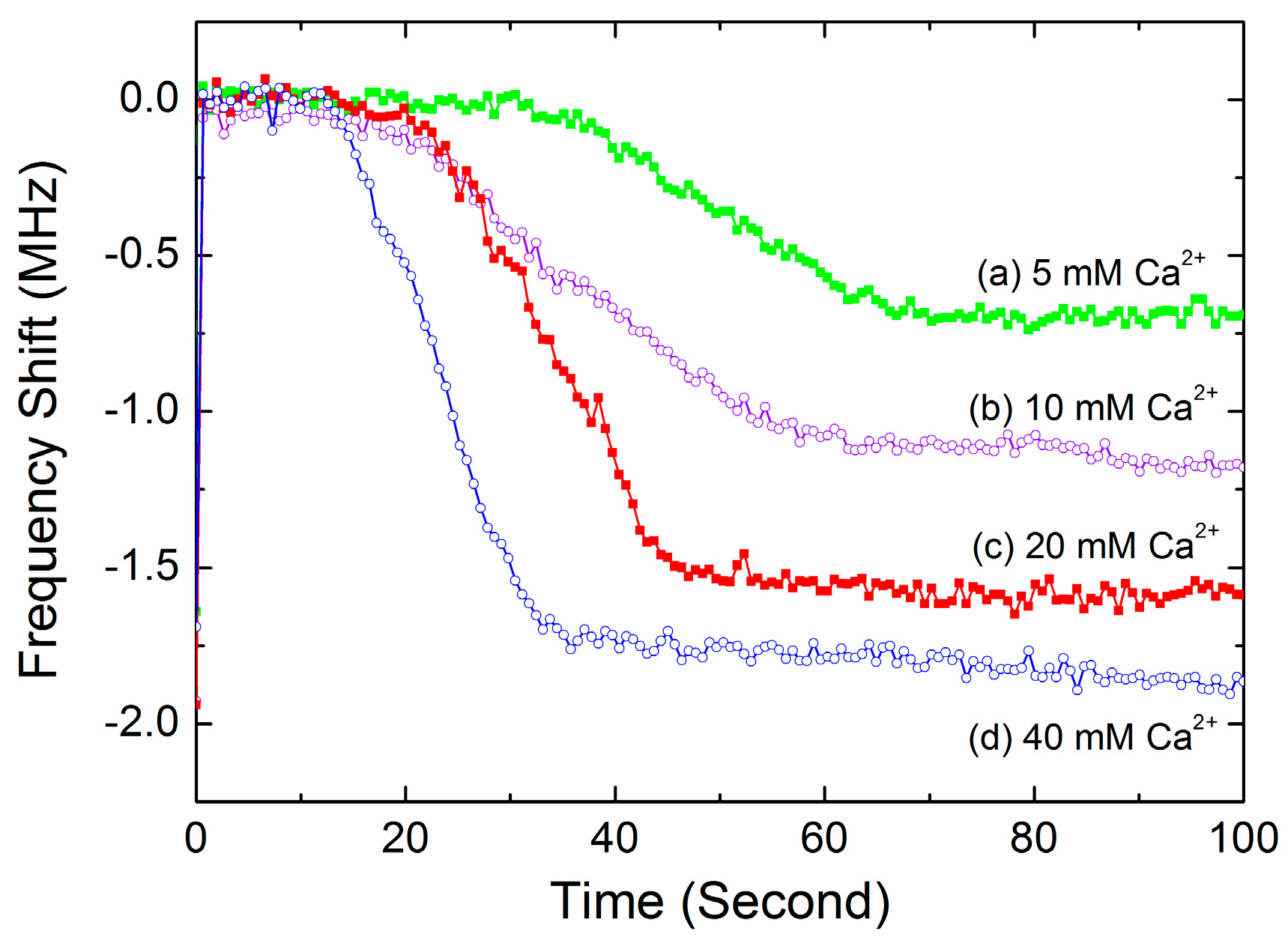
3.3. The Coagulation Kinetics and Parameters
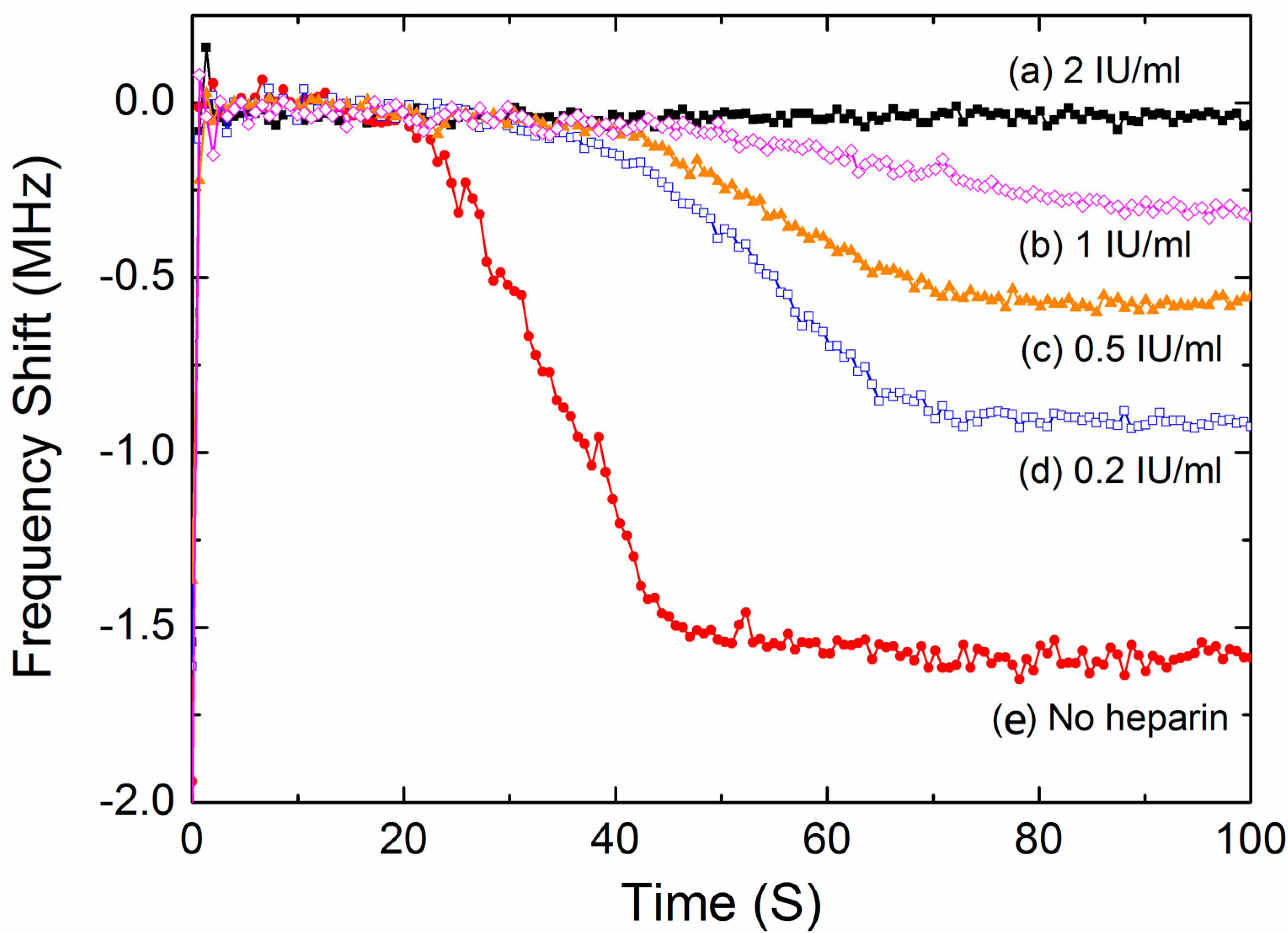
3.4. Test of the Inhibiting Effect of Heparin
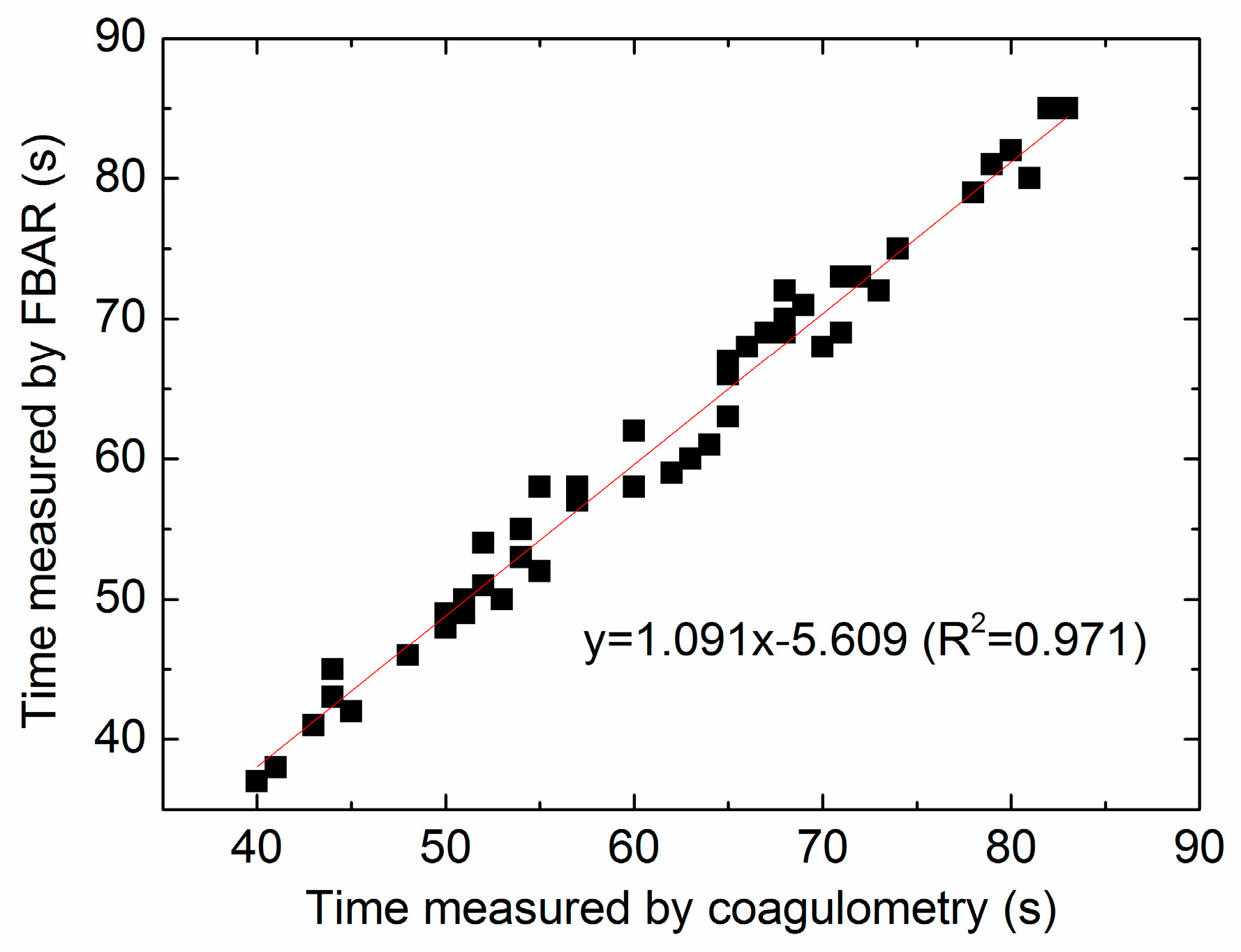
3.5. Comparison between the FBAR Device and Standard Coagulometer
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harris, L.F.; Castro-López, V.; Killard, A.J. Coagulation monitoring devices: Past, present, and future at the point of care. TrAC Trends Anal. Chem. 2013, 50, 85–95. [Google Scholar] [CrossRef]
- Cakmak, O.; Ermek, E.; Kilinc, N.; Bulut, S.; Baris, I.; Kavakli, I. H.; Yaralioglu, G. G.; Urey, H. A cartridge based sensor array platform for multiple coagulation measurements from plasma. Lab Chip 2015, 15, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Padovani, F.; Duffy, J.; Hegner, M. Microrheological Coagulation Assay Exploiting Micromechanical Resonators. Anal. Chem. 2017, 89, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, O.; Elbuken, C.; Ermek, E.; Mostafazadeh, A.; Baris, I.; Erdem Alaca, B.; Kavakli, I.H.; Urey, H. Microcantilever based disposable viscosity sensor for serum and blood plasma measurements. Methods 2013, 63, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Sinn, S.; Drechsel, H.; Ziegler, C.; Wendel, H.P.; Northoff, H.; Gehring, F.K. Investigation of prothrombin time in human whole-blood samples with a quartz crystal biosensor. Anal. Chem. 2010, 82, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Northoff, H.; Gehring, F.K. QCM-D providing new horizon in the domain of sensitivity range and information for haemostasis of human plasma. Biosens. Bioelectron. 2015, 66, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M. QCM-D for haemostasis: Current status and future: A review. UK J. Pharm. Biosci. 2016, 4, 121–132. [Google Scholar] [CrossRef]
- Meyer dos Santos, S.; Zorn, A.; Guttenberg, Z.; Picard-Willems, B.; Kläffling, C.; Nelson, K.; Klinkhardt, U.; Harder, S. A novel μ-fluidic whole blood coagulation assay based on Rayleigh surface-acoustic waves as a point-of-care method to detect anticoagulants. Biomicrofluidics 2013, 7, 056502. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M. ‘Argatroban’ Monitoring in Human Plasma: aPTT and PiCT Studies on QCM-D vs. ‘Gold Standard’. UK J. Pharm. Biosci. 2015, 3, 42–48. [Google Scholar] [CrossRef]
- Hussain, M. aPTT: 1st Recognition for human whole blood on QCM-D platform. UK J. Pharm. Biosci. 2015, 3, 49–55. [Google Scholar] [CrossRef]
- Hussain, M. PiCT: 1st Recognition for Human Whole Blood on QCM-D Platform. UK J. Pharm. Biosci. 2015, 3, 1–8. [Google Scholar]
- Hussain, M. Shortened ‘Thrombin Time’ Monitoring on QCM-D: A Better Substitute of 'Gold Standard'. UK J. Pharm. Biosci. 2016, 4, 20–26. [Google Scholar] [CrossRef]
- Hussain, M.; Sinn, S.; Zeilinger, M.; Northoff, H.; Lieberzeit, P.A.; Gehring, F.K. Blood Coagulation Thromboplastine Time Measurements on a Nanoparticle Coated Quartz Crystal Microbalance Biosensor in Excellent Agreement with Standard Clinical Methods. J. Biosens. Bioelectron. 2013, 4, 139. [Google Scholar]
- Hussain, M. A simultaneous monitoring of coagulation time and fibrinogen via PiCT on QCM-D. UK J. Pharm. Biosci. 2016, 4, 27–35. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Luo, J.K.; Du, X.Y.; Flewitt, A.J.; Li, Y.; Markx, G.H.; Walton, A.J.; Milne, W.I. Recent developments on ZnO films for acoustic wave based bio-sensing and microfluidic applications: A review. Sens. Actuators B 2010, 143, 606–619. [Google Scholar] [CrossRef]
- Pang, W.; Zhao, H.; Kim, E.S.; Zhang, H.; Yuc, H.; Hu, X. Piezoelectric microelectromechanical resonant sensors for chemical and biological detection. Lab Chip 2012, 12, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Mastromatteo, U.; Villa, F.F. High sensitivity acoustic wave AlN/Si mass detectors arrays for artificial olfactory and biosensing applications: A review. Sens. Actuators B 2013, 179, 319–327. [Google Scholar] [CrossRef]
- Katardjiev, I.; Yantchev, V. Recent developments in thin film electro-acoustic technology for biosensor applications. Vacuum 2012, 86, 520–531. [Google Scholar] [CrossRef]
- Lee, S.; Mortazawi, A. An Intrinsically Switchable Ladder-Type Ferroelectric BST-on-Si Composite FBAR Filter. IEEE Trans. Ultrason. Free 2016, 63, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Zhang, D.; Zhang, H.; Pang, W.; Duan, X. Detection of Volatile Organic Compounds by Self-assembled Monolayer Coated Sensor Array with Concentration-independent Fingerprints. Sci. Rep. 2016, 6, 23970. [Google Scholar] [CrossRef] [PubMed]
- Penza, M.; Aversa, P.; Cassano, G.; Suriano, D.; Wlodarski, W.; Benetti, M.; Cannatà, D.; Pietrantonio, F.D.; Verona, E. Thin-Film Bulk-Acoustic-Resonator Gas Sensor Functionalized With a Nanocomposite Langmuir-Blodgett Layer of Carbon Nanotubes. IEEE Trans. Electron. Devices 2008, 55, 1237–1243. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.J.; Li, D.H.; Xu, Y. Hydrogen sensor based on Pd-functionalized film bulk acoustic resonator. Sens. Actuators B 2011, 159, 234–237. [Google Scholar] [CrossRef]
- Bai, P.R.; Liu, Q.Y.; Li, L.; Teng, S.H.; Li, J.; Cao, M.Y. A novel region-based level set method initialized with mean shift clustering for automated medical image segmentation. Comput. Biol. Med. 2013, 43, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pan, F.; Ashley, G.M.; Garcia-Gancedo, L.; Luo, J.; Flewitt, A.J.; Milne, W.I.; Lu, J.R. Label-free detection of human prostate-specific antigen (hPSA) using film bulk acoustic resonators (FBARs). Sens. Actuators B 2014, 190, 946–953. [Google Scholar] [CrossRef]
- Wang, J.; Chenb, D.; Xub, Y.; Liu, W. Label-free immunosensor based on micromachined bulk acoustic resonator for the detection of trace pesticide residues. Sens. Actuators B 2014, 190, 378–383. [Google Scholar] [CrossRef]
- Enlund, J.; Martin, D.M.; Yantchev, V.; Katardjiev, I. FBAR Sensor Array for in Liquid Operation. IEEE Sens. J. 2012, 10, 1903–1904. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Chen, D.; Xu, Y.; Zhang, L. A micro-machined thin film electro-acoustic biosensor for detection of pesticide residuals. J. Zhejiang Univ. Sci. C 2014, 15, 383–389. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Xu, Y.; Li, D. A pure shear mode ZnO film resonator for the detection of organophosphorous pesticides. Sens. Actuators B 2012, 171, 1081–1086. [Google Scholar] [CrossRef]
- Wingqvist, G.; Bjurstroöm, J.; Liljeholm, L.; Yantchev, V.; Katardjiev, I. Shear mode AlN thin film electro-acoustic resonant sensor operation in viscous media. Sens. Actuators B 2007, 123, 466–473. [Google Scholar] [CrossRef]
- Wingqvist, G.; Anderson, H.; Lennartsson, C.; Weissbach, T.; Yantchev, V.; Lloyd Spetz, A. On the applicability of high frequency acoustic shear mode biosensing in view of thickness limitations set by the film resonance. Biosens. Bioelectron. 2009, 24, 3387–3390. [Google Scholar] [CrossRef] [PubMed]
- Fardeheb-Mammeri, A.; Assouar, M.B.; Elmazria, O.; Gatel, C.; Fundenberger, J.J.; Benyoucef, B. C-axis inclined AlN film growth in planar system for shear wave devices. Diamond Relat. Mater. 2008, 17, 1770–1774. [Google Scholar] [CrossRef]
- Link, M.; Weber, J.; Schreiter, M.; Wersing, W.; Elmazria, O.; Alnot, P. Sensing characteristics of high-frequency shear mode resonators in glycerol solutions. Sens. Actuators B 2007, 121, 372–378. [Google Scholar] [CrossRef]
- Li, X.-Y.; Li, Y.-X.; Yang, H.-X. Two families of Liouville integrable lattice equations. Appl. Math. Comput. 2011, 217, 8671–8682. [Google Scholar] [CrossRef]
- Zhang, H.; Marma, M. S.; Kim, E.S.; McKenna, C.E.; Thompson, M.E. A film bulk acoustic resonator in liquid environments. J. Micromech. Microeng. 2005, 15, 1911–1916. [Google Scholar] [CrossRef]
- Lu, F.; Zeng, Q.; Duan, H. Synchronization-Core-Based Discovery of Processes with Decomposable Cyclic Dependencies. ACM Trans. Knowl. Discov. Data 2016, 10, 1–29. [Google Scholar] [CrossRef]
- Xu, W.C.; Appel, J.; Chae, J. Real-Time Monitoring of Whole Blood Coagulation Using a Microfabricated Contour-Mode Film Bulk Acoustic Resonator. J. Microelectromech. Syst. 2012, 21, 302–307. [Google Scholar] [CrossRef]
- Chen, D.; Song, S.; Ma, J.; Zhang, Z.; Wang, P.; Liu, W.; Guo, Q. Micro-electromechanical film bulk acoustic sensor for plasma and whole blood coagulation monitoring. Biosens. Bioelectron. 2017, 91, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Graveline, A.; Waterhouse, A.; Vernet, A.; Flaumenhaft, R.; Ingber, D.E. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat. Commun. 2016, 7, 10176. [Google Scholar] [CrossRef] [PubMed]
- Nirschl, M.; Rantala, A.; Tukkiniemi, K.; Auer, S.; Hellgren, A.C.; Pitzer, D.; Schreiter, M.; Vikholm-Lundin, I. CMOS-integrated film bulk acoustic resonators for label-free biosensing. Sensors 2010, 10, 4180–4193. [Google Scholar] [CrossRef] [PubMed]


| Parameters | In Air | In Water | In Blood |
|---|---|---|---|
| fs (GHz) | 1.430 | 1.426 | 1.418 |
| fp (GHz) | 1.441 | 1.437 | 1.429 |
| Qs | 430 | 404 | 376 |
| Qp | 433 | 407 | 379 |
| C0 (pF) | 2.73 | 2.69 | 2.63 |
| Cm (fF) | 44.8 | 44.2 | 43.3 |
| Lm (μH) | 27.6 | 28.2 | 30.4 |
| Rm (Ω) | 5.78 | 6.21 | 7.22 |
| Ca2+ Concentrations (mM) | Enzymatic Cascade Time (s) | Coagulation Rate Constant k | Finial Frequency Downshift Δf (MHz) | PT (s) | CV of PT |
|---|---|---|---|---|---|
| 5 | 30 ± 2.0 | 0.13 ± 0.006 | 0.79 ± 0.06 | 64 ± 5.5 | 8.5% |
| 10 | 23 ± 1.8 | 0.17 ± 0.021 | 1.21 ± 0.08 | 58 ± 4.8 | 8.2% |
| 20 | 18 ± 1.1 | 0.18 ± 0.012 | 1.63 ± 0.09 | 52 ± 3.0 | 5.7% |
| 40 | 15 ± 2.0 | 0.22 ± 0.020 | 1.92 ± 0.15 | 42 ± 2.4 | 5.7% |
| Heparin Concentrations (IU/mL) | Enzymatic Cascade Time (s) | Coagulation Rate Constant k | Finial Frequency Downshift Δf (MHz) | PT (s) | CV of PT |
|---|---|---|---|---|---|
| 0 | 18 ± 1.1 | 0.18 ± 0.012 | 1.63 ± 0.09 | 52 ± 3.0 | 5.7% |
| 0.2 | 35 ± 2.4 | 0.15 ± 0.012 | 0.91 ± 0.04 | 70 ± 4.0 | 5.7% |
| 0.5 | 41 ± 3.6 | 0.12 ± 0.008 | 0.58 ± 0.02 | 72 ± 4.3 | 6.0% |
| 1 | 46 ± 3.8 | 0.08 ± 0.004 | 0.29 ± 0.04 | 80 ± 7.4 | 9.3% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Zhang, Z.; Ma, J.; Wang, W. ZnO Film Bulk Acoustic Resonator for the Kinetics Study of Human Blood Coagulation. Sensors 2017, 17, 1015. https://doi.org/10.3390/s17051015
Chen D, Zhang Z, Ma J, Wang W. ZnO Film Bulk Acoustic Resonator for the Kinetics Study of Human Blood Coagulation. Sensors. 2017; 17(5):1015. https://doi.org/10.3390/s17051015
Chicago/Turabian StyleChen, Da, Zhen Zhang, Jilong Ma, and Wei Wang. 2017. "ZnO Film Bulk Acoustic Resonator for the Kinetics Study of Human Blood Coagulation" Sensors 17, no. 5: 1015. https://doi.org/10.3390/s17051015





